Question
Question: Which of the following will not produce ethane? A) Reduction of \(C{H_3}COOH\) with HI and red P ...
Which of the following will not produce ethane?
A) Reduction of CH3COOH with HI and red P
B) Reduction of CH3COCH3 with HI and red P
C) Soda-lime decarboxylation of sodium propionate
D) Hydrogenation of ethene in the presence of Raney- Ni
Solution
We will check each reaction to find the best suitable answer. Also, we should know the reagent behavior if it is reducing oxidation. Here HI and P are reducing agents. Raney Ni acts as a catalyst and reduces the alkene to form an alkane hydrocarbon.
Complete answer:
To solve this problem we will by each reaction-
A) Reduction of CH3COOH with HI and red P-
We know that red P and HI act as reducing agents. So this reagent will reduce acetic acid into ethane. This is how the reaction will proceed.
CH3COOH+6HIRed PCH3−CH3+2H2O+3I2
So we can say that reduction of acetic acid with rep P and HI produced ethane.
B) Reduction of CH3COCH3 with HI and red P-
The reaction of aldehyde and ketone with red P and HI is as follows-
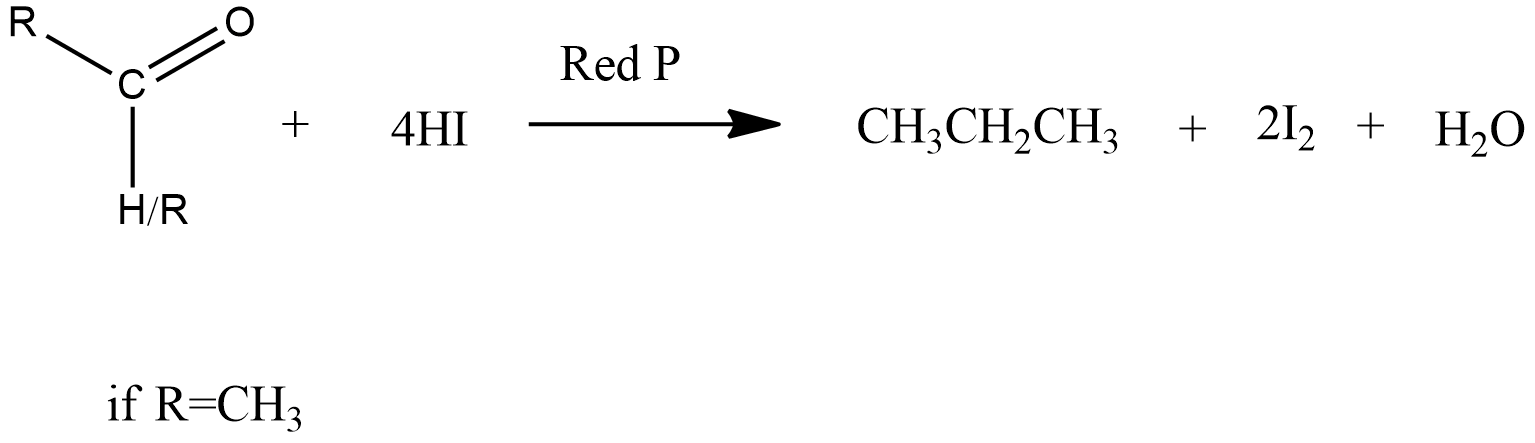
If aldehyde reacts with red P and HI then we get ethane. But in this reaction, we get propane, not ethane.
C) Soda-lime decarboxylation of sodium propionate –
This is the decarboxylation reaction and in this reaction, ethane will liberate. The reaction is as follows:
C2H5COONa+NaOHCaONa2CO3+C2H6↑
Here in this reaction also ethane is produced.
D) Hydrogenation of ethene in the presence of Raney- Ni –
In hydrogenation reactions H2 will be attached to unsaturated hydrocarbons in the presence of catalytic Ni/Pd. We get alkanes.
CH2CH2+H2NiCH3CH3
In this reaction they also get ethane.
Hence we can say that the correct answer is option (B).
Note:
Reduction of CH3COCH3 with HI and red P in this reaction is slightly different which makes confusion. If we take acetaldehyde instead of acetone then we will get ethane as a product. But here we have acetone so it will give us propane, not ethane. Do the reaction carefully.
