Question
Question: Which of the following will not give Hoffmann bromamide reaction? 
Solution
The reaction in which an amide is converted into an amine having one carbon atom less is known as the Hoffmann Bromamide reaction. This reaction is given by both alkyl and aryl amides but they must be primary amides.
Complete step by step answer:
The reaction in which primary amide (either alkyl or aryl) is treated with an aqueous or ethanolic solution of potassium hydroxide and bromine forms a primary amine, but this amine has one carbon less than the carbon atoms in the amide. So this can only be given by primary amides. So we have to check which compound doesn’t have primary amide. Primary amides are those in which the nitrogen has two hydrogen atoms, secondary amides are those in which the nitrogen has one hydrogen atom and tertiary amides are those in which the nitrogen doesn't have a hydrogen atom.
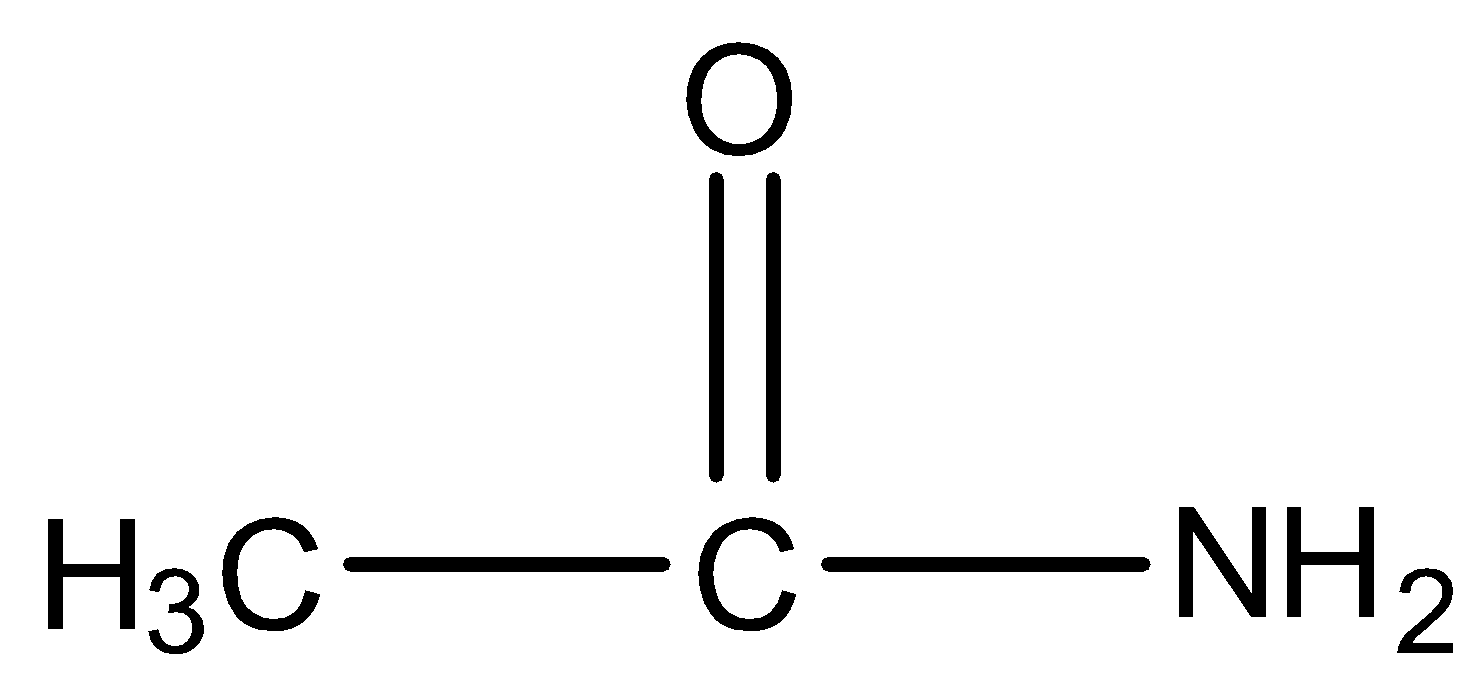
In this molecule, the amide is primary because the nitrogen has two hydrogen atoms so it will give Hoffmann Bromamide reaction.
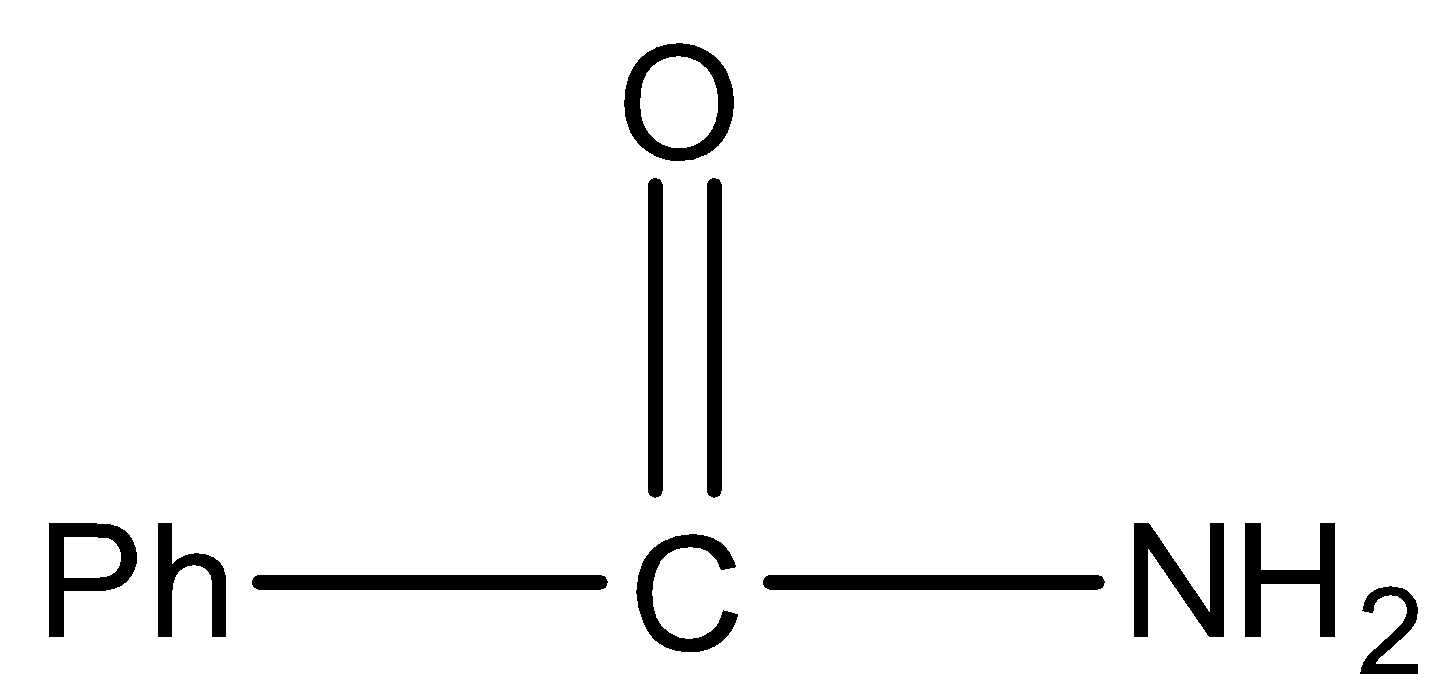
In this molecule, the amide is primary because the nitrogen has two hydrogen atoms so it will give Hoffmann Bromamide reaction.
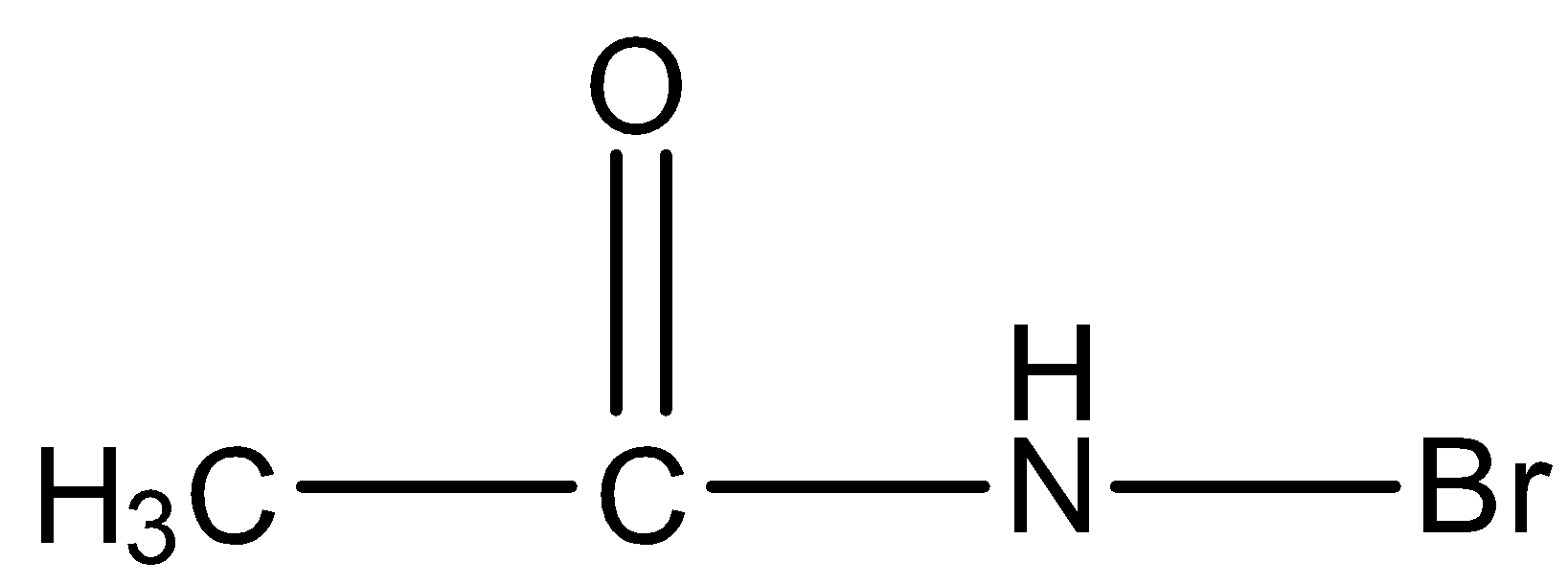
In this molecule, the amide is primary because the nitrogen has one hydrogen atom and one bromine atom and it is an intermediate in the Hoffmann bromamide reaction, so it will give Hoffmann Bromamide reaction.
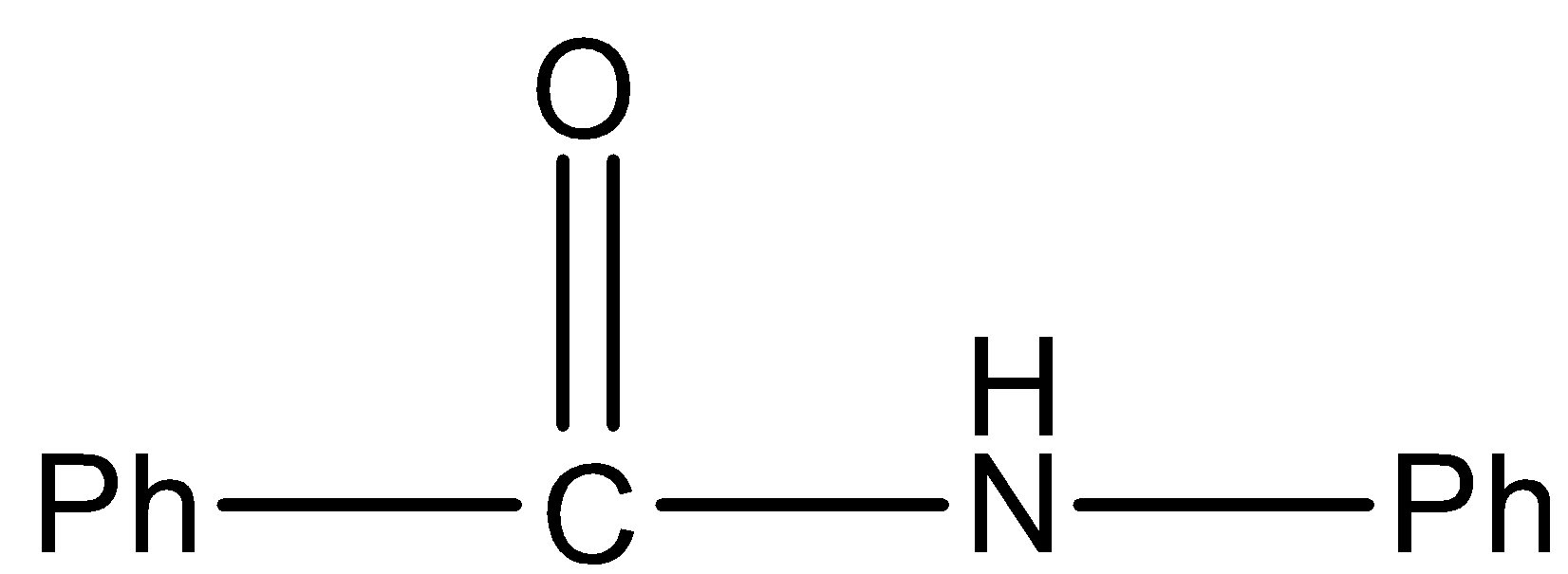
In a molecule, the amide is secondary because the nitrogen atom has only one hydrogen atom so it will not give Hoffmann bromamide reaction.
Therefore, the correct answer is an option (d).
Note: The general reaction of Hoffmann bromamide reaction is given below:
R−CONH2+Br2+4KOH→R−NH2+K2CO3+2KBr+2H2O. In general, this reaction is used for producing amine having one carbon atom less than the reactant.
