Question
Question: Which of the following will not give an iodoform test? A. Ethanal B. Ethanol C. \( 2 \) - prop...
Which of the following will not give an iodoform test?
A. Ethanal
B. Ethanol
C. 2 - propanone
D. 3 - pentanone
Solution
In order to answer this question, we should know about the iodoform test. Basically the iodoform test is used to determine the presence of the carbonyl group in the compound. In this test, iodine reacts with a base and the carbonyl compound which will give yellow colour precipitate.
Complete answer:
Iodoform test is used to determine the presence of the carbonyl compound with the structure, CH3−C=O and the secondary alcohol. In this test, basically iodine reacts with a base and the carbonyl compound to form a yellow colour precipitate which has the smell like antiseptic.
The Iodoform test comes out to be positive if the required function group is present in it i.e. CH3−C=O .
In the given question, four compounds are given and we need to find that compound which will not give an iodoform test. We can determine by looking at the structure of each compound and the compound which do not contain the functional group i.e. CH3−C=O do not give the iodoform test.
Let’s see the structures of all the given compounds.
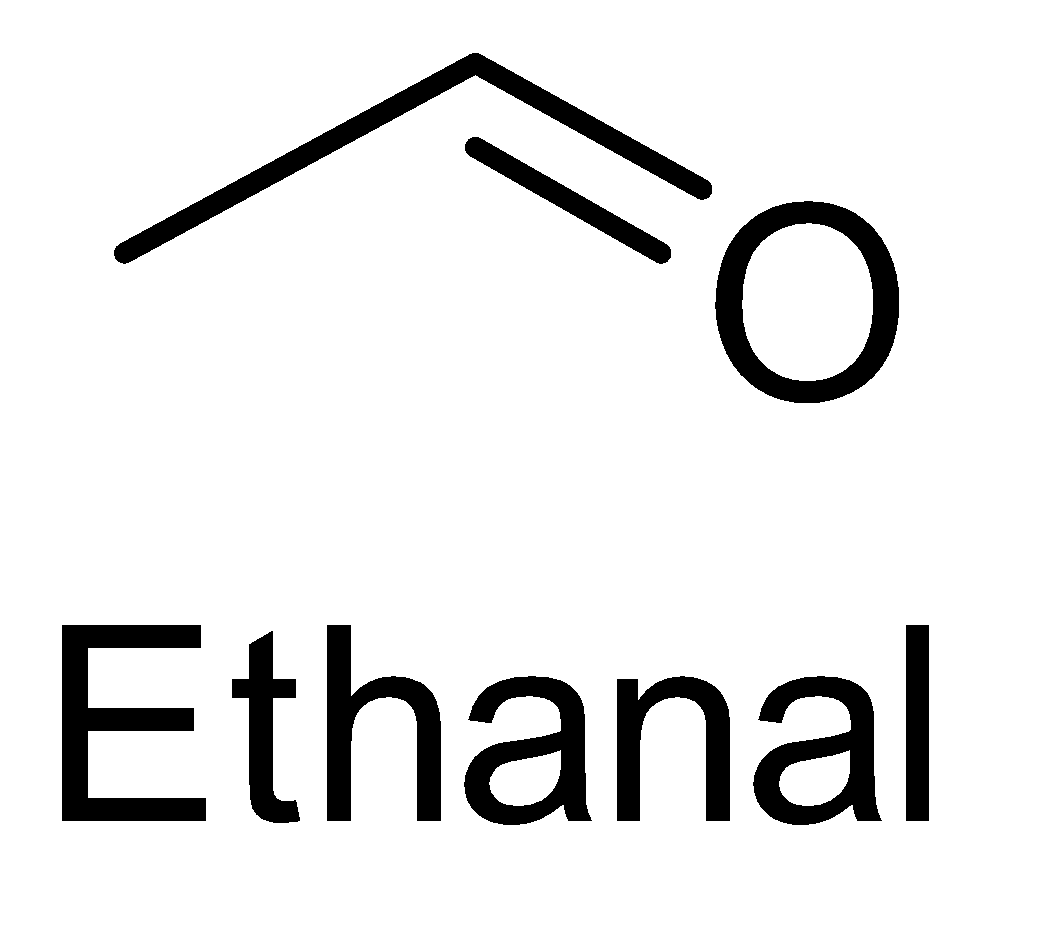
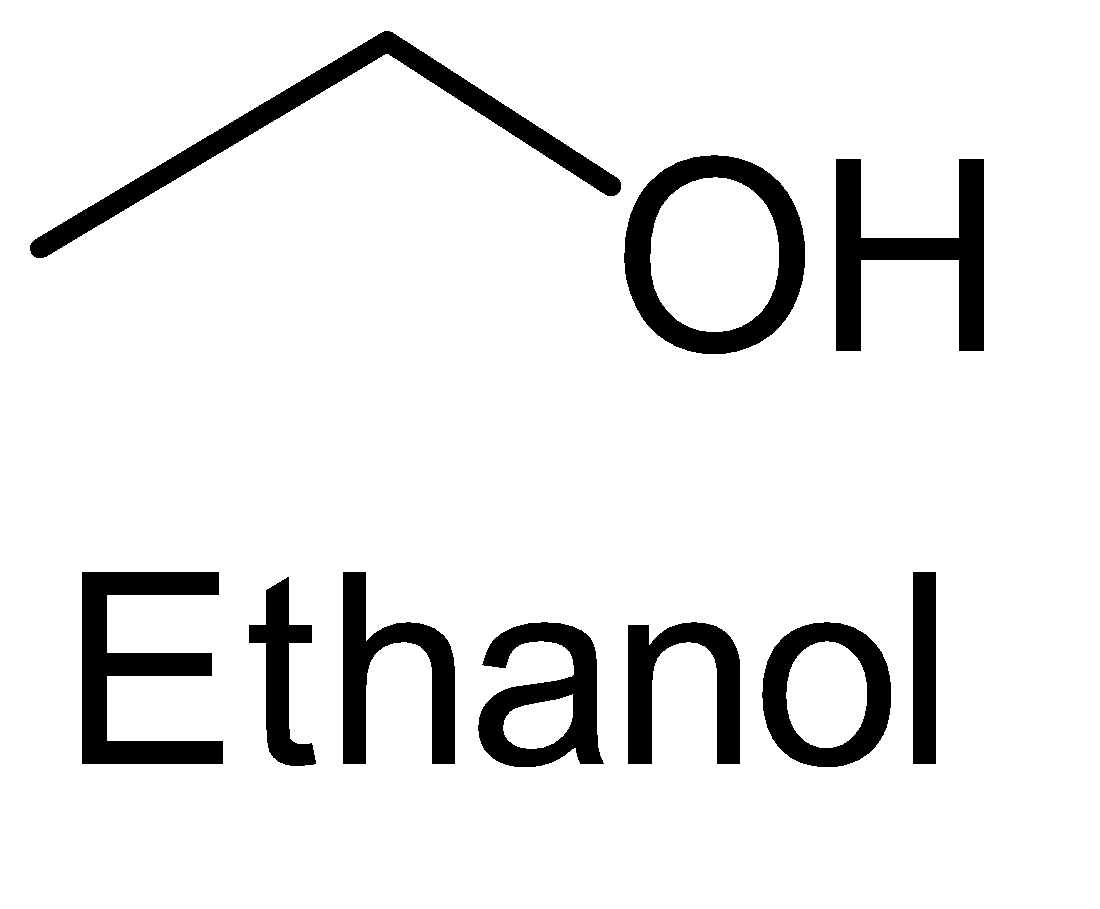
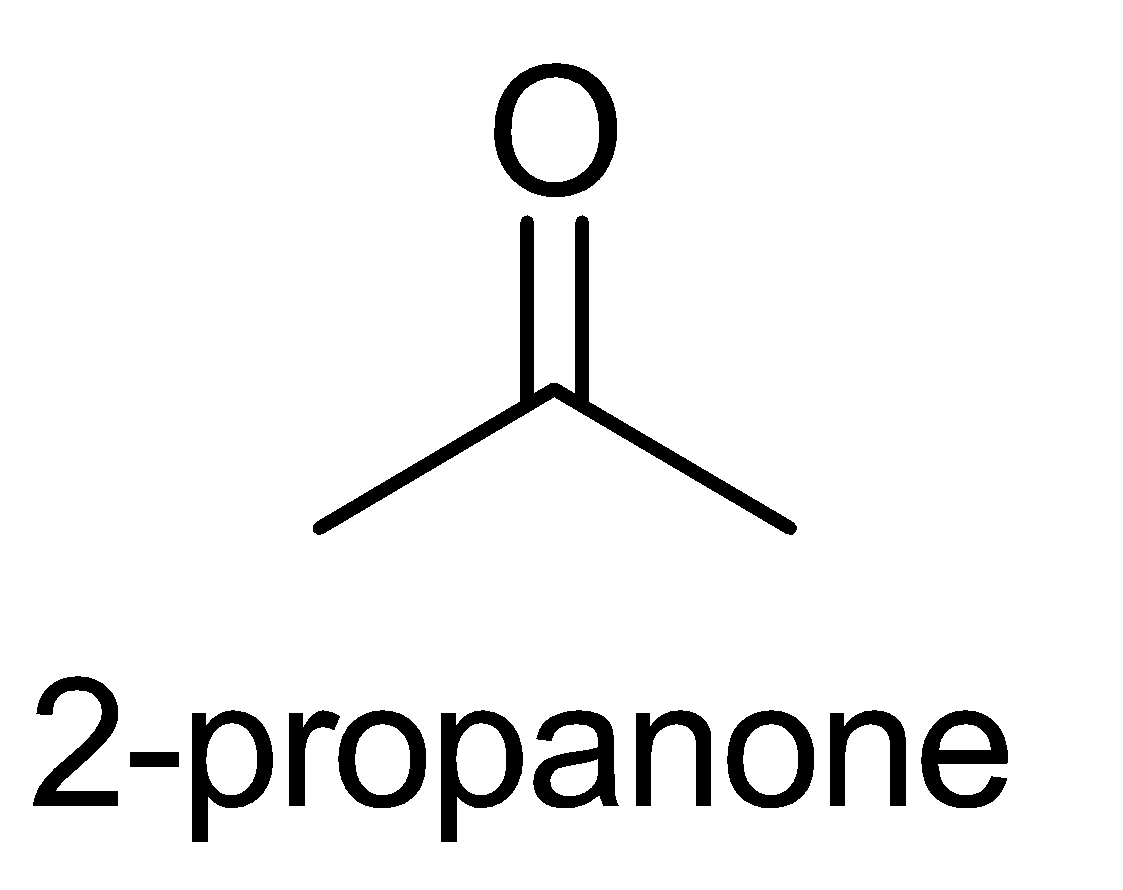
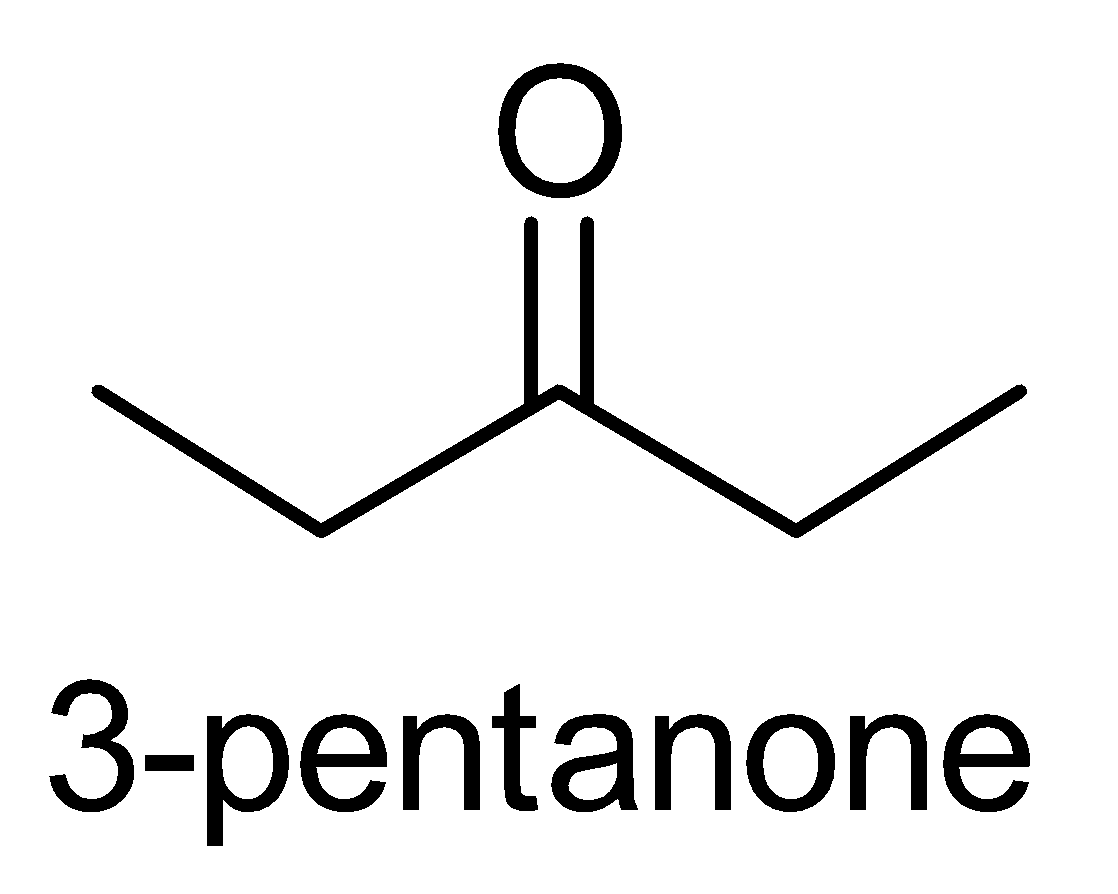
The last compound i.e. 3 - pentanone does not contain the required functional group with the structure CH3−C=O for the iodoform test.
Therefore, 3 - pentanone does not give the iodoform test.
The correct answer is option (D).
Note:
It must be remembered that primary alcohols do not test for iodoform test. Ethanol is the only primary alcohol which gives the iodoform test. Also, the only aldehyde which will give the iodoform test is the acetaldehyde because it contains the desired functional group i.e. CH3−C=O .
