Question
Question: Which of the following will not be able to show optical isomerism (enantiomerism)? A. I,2-Propadie...
Which of the following will not be able to show optical isomerism (enantiomerism)?
A. I,2-Propadiene
B. 2,3-Pentadiene
C. Sec-Butyl alcohol
D. All exhibit enantiomerism
Solution
Now the molecule, which is achiral, cannot show optical isomerism (enantiomerism) and achiral molecule is a molecule which is superimposed on its mirror image. Draw the structure of all the four options given above and see whichever molecule is achiral will not show optical isomerism (enantiomerism).
Complete step by step solution:
In chemistry there is a basic term isomerism. Isomerism occurs when certain compounds, having seen molecular formulas differ in the arrangement of their structure. There are different kinds of isomerism; one such isomerism is known as stereoisomerism. In stereoisomerism, compounds have the same molecular formula and same structure formula. But they differ in the arrangement of their 3D structure that is rotation of the bond.
Now, stereoisomerism contains another term that is called optical isomerism. In this, compounds having the same molecular formula have the same structure formula, but they only differ when a polarized light fall on them. The direction in which the polarized light rotates is left or right, that defers the compound.
Now we will draw the structure of the three options given above to see which of them generates a optical isomer (enantiomerism)
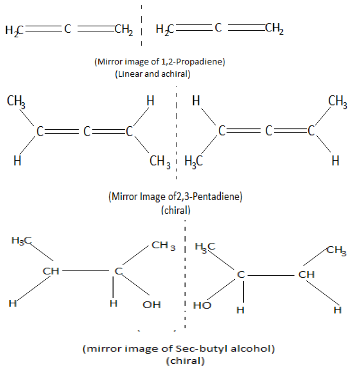
Thus the correct answer to the given question is option A.
Note: Remember that if the structure of a molecule is linear it is always achiral, that is, it's always super imposable on its mirror image. Molecules which have non-linear structure can be a chiral molecule. Also, achiral molecules do not create optical isomers.
