Question
Question: Which of the following will have metal deficiency defects? (A) \(NaCl\) (B) \(FeO\) (C) \(KCl...
Which of the following will have metal deficiency defects?
(A) NaCl
(B) FeO
(C) KCl
(D) ZnO
Solution
This type of defect occurs in compounds where the metal can exhibit variable valency or variable oxidation state. e.g., Transition metal compounds. Out of given options in the question Zn and Fe are transition elements. But Fe can show variable oxidation state.
Complete step by step answer:
In a metal deficiency defect, from its lattice site the cation is missing. Metal deficiency defect is may be due to missing of a cation of lower valency and presence of a cation of higher valency. The nearest metal ion acquires an extra positive charge for maintaining electrical neutrality. In the given options, the only metal that exhibits a variable valency is Fe. Fe can exhibit +2 oxidation state as well as +3 oxidation state.
When the conversion of ferrous to ferric ion takes place, charge gets compensated.
Fe2++2e−→Fe
For every vacancy of iron cation, two ferrous ions are converted into ferric ions by providing the two-electrons required by excess of oxygen.
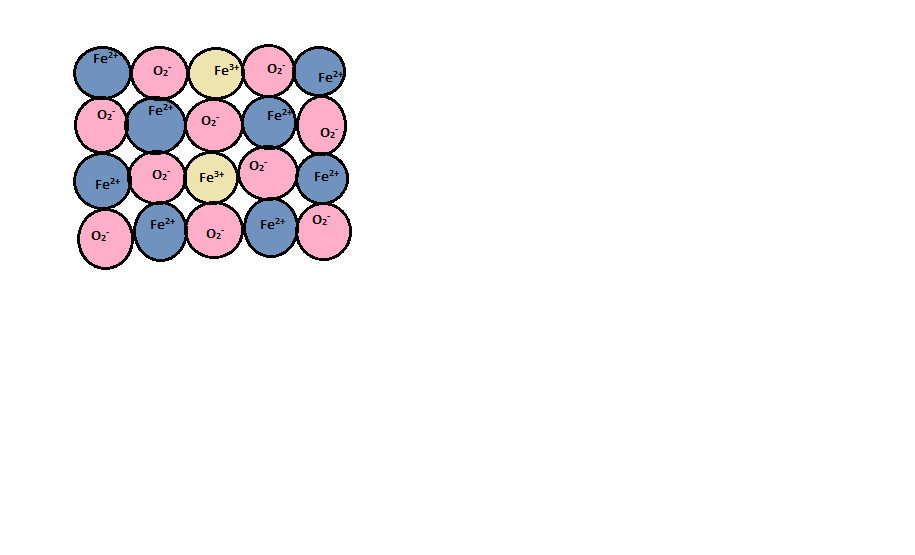
So, FeO is the correct answer.
Thus, option (D) is the correct answer.
Note:
There are two types of non-stoichiometric defect, one is metal excess defect and another one is metal deficiency defect. In a metal deficiency defects, density of solids decreases while in metal excess defects, density of solids increases.
