Question
Question: Which of the following will have a meso isomer also? A.Cis-1,2dichlorocyclobutane B.2,3-dichloro...
Which of the following will have a meso isomer also?
A.Cis-1,2dichlorocyclobutane
B.2,3-dichlorobutane
C.2,2-dichlorobutane
D.2,3-dimethylbutane
Solution
Basically, mesomers are the types of organic compounds in which two chiral carbons are present and those two are further similar. Moreover, the net rotation of plane polarized light is zero for these compounds.
Complete step by step answer:
Stereochemistry is the branch of chemistry that involves the study of the different spatial arrangements of atoms in molecules. Basically, a meso compound or meso isomer is a non-optically active member of a set of stereoisomers and at least two of which are optically active. They are achiral compounds that have multiple chiral centers. In general, a meso compound should contain two or more identical substituted stereo centers. Moreover, it has an internal symmetry plane that divides the compound in half and these two halves reflect each other by the internal mirror.
Further, these compounds can exist in many different forms such as pentane, butane, heptane and even cyclobutane. They can have more than two stereocenters.
Now, from the given options is-1,2-dichlorocyclobutane and 2,3-dichlorobutane, both have the meso isomers as compared to the rest of the options as they contain a plane of symmetry and are superimposable on their mirror images. Their structures are given below:
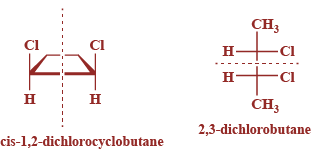
Hence, option A and B are correct.
Note:
In case of single bonds and sp3 orbitals, we can rotate the substituted group that is attached to a stereo center to recognize the internal plane. So, as the molecule is rotated, its stereochemistry does not change. Besides meso compounds, there are other types of molecules such as diastereomer, enantiomer etc.
