Question
Question: Which of the following will have a meso isomer also? (This question has multiple answers.) a) \(...
Which of the following will have a meso isomer also?
(This question has multiple answers.)
a) Cis1,2−dichlorocyclobutane
b) 2,3−Dichlorobutane
c) 2,2−Dichlorobutane
d) 2,3−Dimethylbutane
Solution
Proper knowledge about optical isomerism is necessary. Meso compounds are those which either have an internal plane of symmetry or they have a centre of symmetry inside the structure of the molecule.
Complete step by step answer
Cis and Trans isomers are those where the vicinal substituents are attached on the same side and on the opposite sides respectively.
Chiral carbons are those carbons which have four different substituent groups. These types of carbon centres cause optical nature in some compounds. Some compounds can have one or more than one chiral centres.
Optical isomers are those pairs of isomers which have the same substituent groups but the spatial arrangements of those groups will be different.
To be a meso compound, it must have at least two chiral centres.
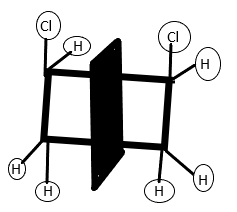
In the first molecule i.e.Cis1,2−dichlorocyclobutane, the two chlorine groups are on the same side. Thus there is an internal plane of symmetry (represented by the broad black line) present in the molecule. Because of this the molecule is not optically active and called a meso compound.
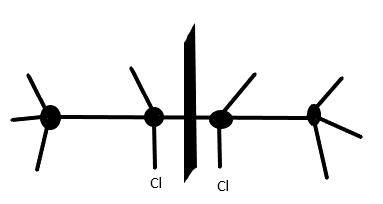
2nd molecule 2,3−Dichlorobutane also has an internal plane of symmetry as the two chlorine groups are on the same side which restricts the optical activity and makes the compound meso.
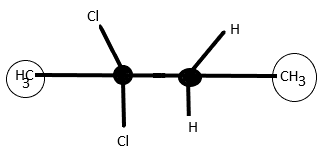
3rd molecule 2,2−Dichlorobutane has no chiral centre as the same atoms are present at carbon number 2 and 3, thus meso is not possible.
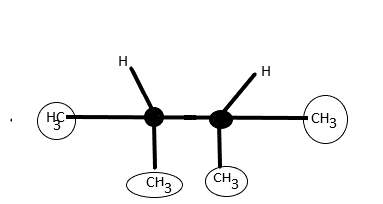
4th molecule 2,3−Dimethylbutane has two chiral centres as they do not contain carbon centres with four different substituents. Thus meso is not possible here.
Hence the correct options are (a) and (b).
Note: To be in the criteria of meso compound it is necessary to have at least two chiral centres. Also meso compounds do not have optical activity. But when calculating stereoisomers, meso compounds are also taken into account.
