Question
Question: Which of the following will give yellow precipitate with KOI? a.) Cyclopentyl methyl carbinol b....
Which of the following will give yellow precipitate with KOI?
a.) Cyclopentyl methyl carbinol
b.) α -phenyl ethanol
c.) AAE
d.) I3C−CHO
Solution
The compound (or answer to this question) reacts with KOI (base), to give a yellow precipitate. This is a distinction test for compounds containing a group, known as the alpha-methyl group.
Complete step by step solution:
The reaction in which a compound on reaction with a base (in this case, KOI) gives a yellow precipitate is an example of iodoform reaction. This is a type of haloform reaction.
This type of a test is specifically shown by a compound that contains an alpha-methyl group. This test is mainly given by aldehydes and ketones.
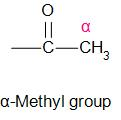
Let us draw all the compounds given in the question and check it for the ‘alpha-methyl group’.
Option (a) Cyclopentyl methyl carbinol
This compound does not contain an alpha-methyl group.
The structure of this compound is –
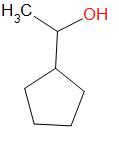
It does not give an iodoform reaction.
Option (b) α -phenyl ethanol
This compound does not contain an alpha-methyl group.
The structure of this compound is –
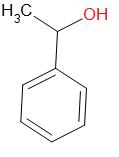
It does not give an iodoform reaction.
Option (c) AAE
This compound is also known as Aceto-acetic ester.
This compound contains the alpha-methyl group.
The structure of this compound is –
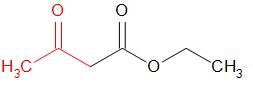
Therefore, this compound gives a positive iodoform test.
Option (d) I3C−CHO
This compound does not contain an alpha-methyl group.
The structure of this compound is –
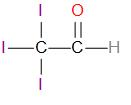
It does not give an iodoform reaction.
Therefore, the answer is – option (c) – AAE.
Additional Information: Iodoform form test is a type of haloform test. Other haloform tests are – chloroform and bromoform.
Note: Iodoform is also known as tri-iodomethane because of its structure - CHI3. It occurs as a pale yellow, crystalline, volatile compound which has a distinctive odour (like antiseptics). Chloroform is a colourless liquid. Bromoform is a colourless liquid with a sweet smell.
