Question
Question: Which of the following will give propyne on hydrolysis? A.\(A{l_4}{C_3}\) B.\(M{g_2}{C_3}\) ...
Which of the following will give propyne on hydrolysis?
A.Al4C3
B.Mg2C3
C.B4C
D.La4C3
Solution
Propyne is an alkyne. For organic synthesis, Propyne is a convenient three-carbon construction block with four hydrogen atoms having a triple bond between two carbon atoms. In order to find the compound which will give propyne on hydrolysis we must write the hydrolysis reaction of each of the compounds given. And from the product obtained you will come to know which compound gives propyne.
Complete step by step answer:
Let us first understand hydrolysis;
Hydrolysis is a chemical reaction where the water molecules are added to the compound undergoing the reaction. During hydrolysis the water molecule undergoes decomposition to give H+ and OH−ions respectively.
HOH⇄H++OH−
All the given options are examples of metal carbides.
Now, we know that carbides are compounds that have carbon and other elements which have less electronegative character than carbon atoms.
Metal carbides are the compounds which have a transition metal ion and carbon atom as the constituents. These structures are more of a complex type. Propyne is basically, C3H4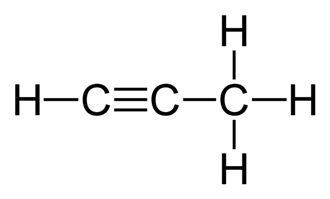
Now, we must perform hydrolysis on the given options to see which compound will give propyne:
In option A, Al4C3 is a metal carbide and on hydrolysis yields methane and aluminium hydroxide.
Al3C4+12HOH→3CH4+4Al(OH)3
So, option A is the incorrect answer.
In option B, Mg2C3 is an ionic carbide and on hydrolysis with water yields magnesium hydroxide and propyne which is a three carbon product.
Mg2C3+4H2O→2Mg(OH)2+C3H4
So, option B is correct.
In option C, B4C on hydrolysis gives boron oxide while carbon monoxide and hydrogen gases are evolved.
B4C+7H2O→2B2O3+CO+7H2
So, option C is the incorrect answer.
In option D, La4C3 on hydrolysis gives lanthanum hydroxide and methane.
La4C3+HOH→La(OH)3+CH4
So, option D is also the incorrect answer.
Thus, from the above chemical reactions it is clear that Mg2C3 is the correct option. As it gives propyne as a product.
Hence, option B is the correct answer to this question.
We should also remember that propyne is also called as allylene.
Note:
Mg2C3 hydrolysis yields a combination of propyne and propadiene, the ratio of which is similar to ambient and higher temperature equilibrium levels, while at low temperatures it changes towards propadiene. The hydrolysis reaction's rigorousness complies well with the structural consequences of polycentre, electron-deficient bonds.
