Question
Question: Which of the following will give phenol with \[{\text{CaO}}\] and \[{\text{NaOH}}\]? A. Salicylic ...
Which of the following will give phenol with CaO and NaOH?
A. Salicylic acid
B. Picric acid
C. Benzoic acid
D. Amino acid
Solution
First we remember that phenol is an aromatic organic compound with the molecular formulaC6H5OH.
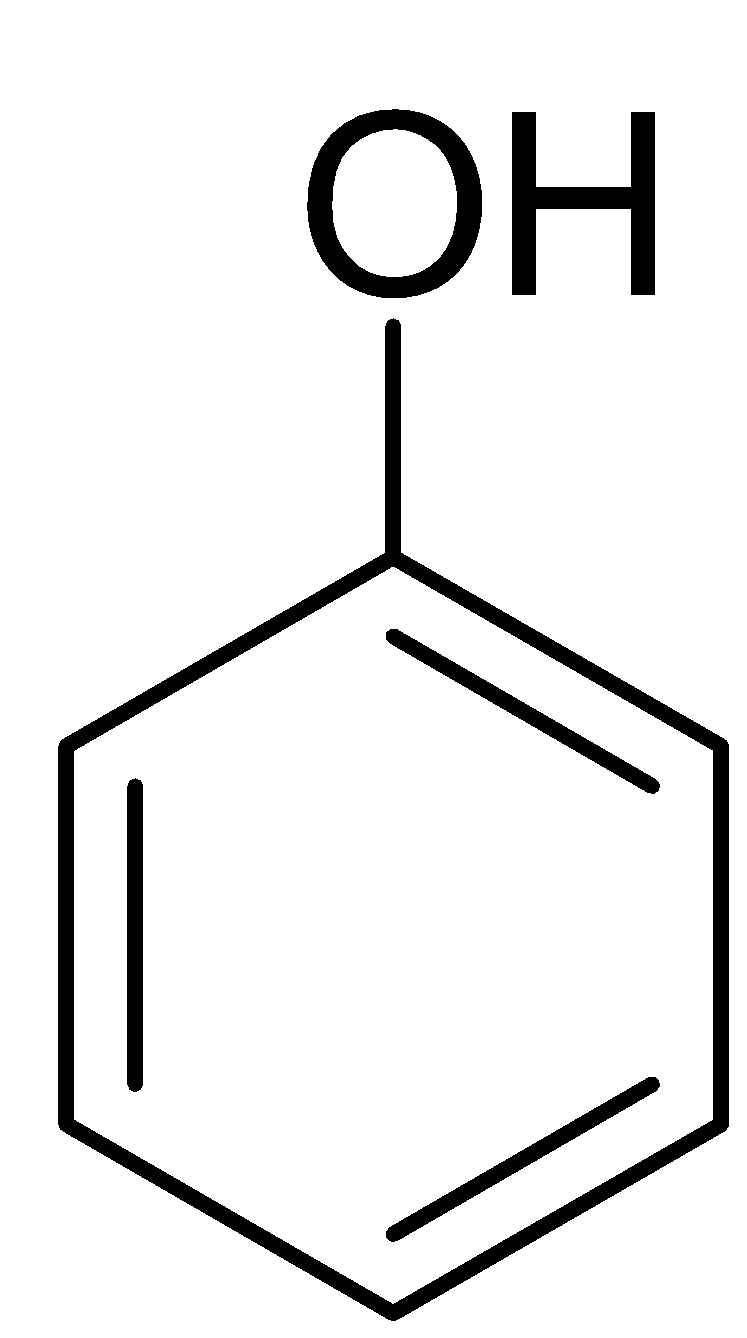
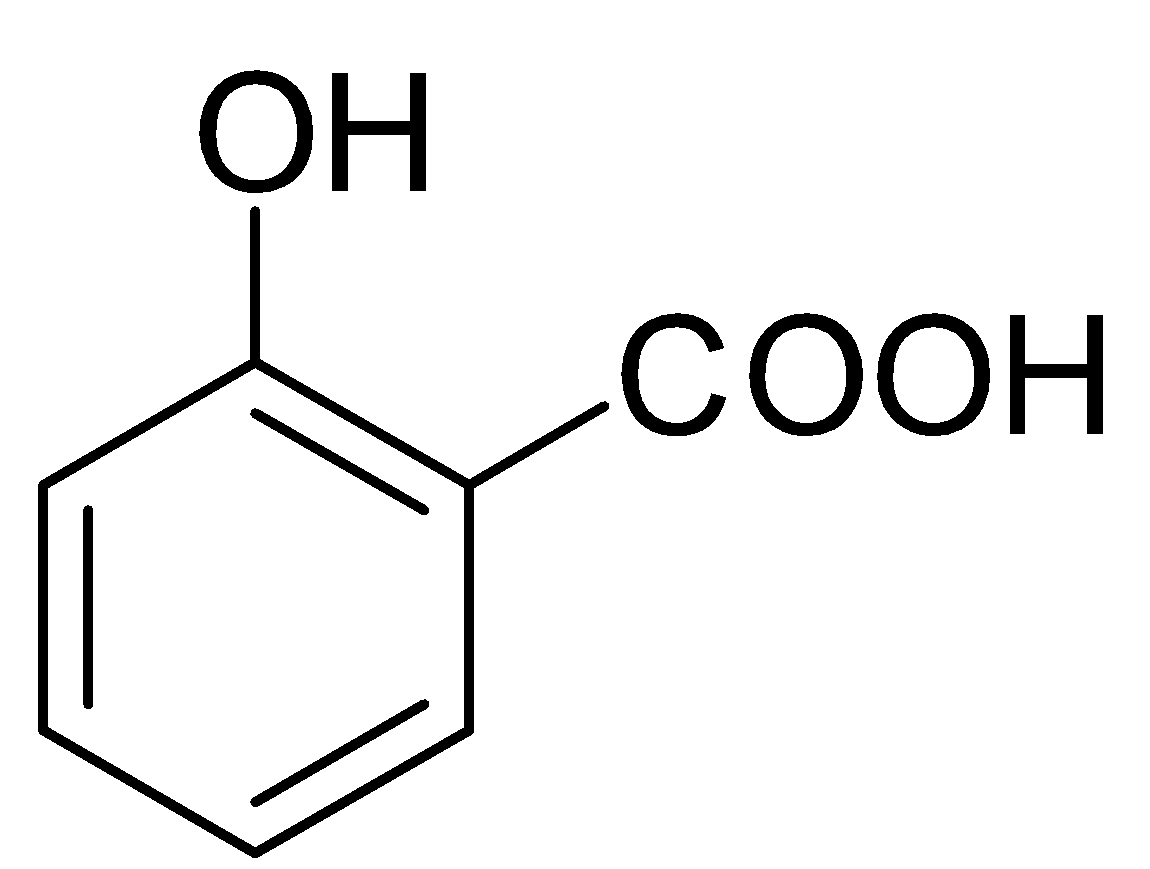
Only salicylic acid (2-Hydroxybenzoic acid) on decarboxylation gives phenol.
Complete answer:
We must know that salicylic acid will give phenol with CaO and NaOH. The mixture of CaO and NaOH is called soda lime. When we heat carboxylic acids with soda lime, they undergo decarboxylation. The by-products in this reaction apart from phenol are sodium carbonate and water.
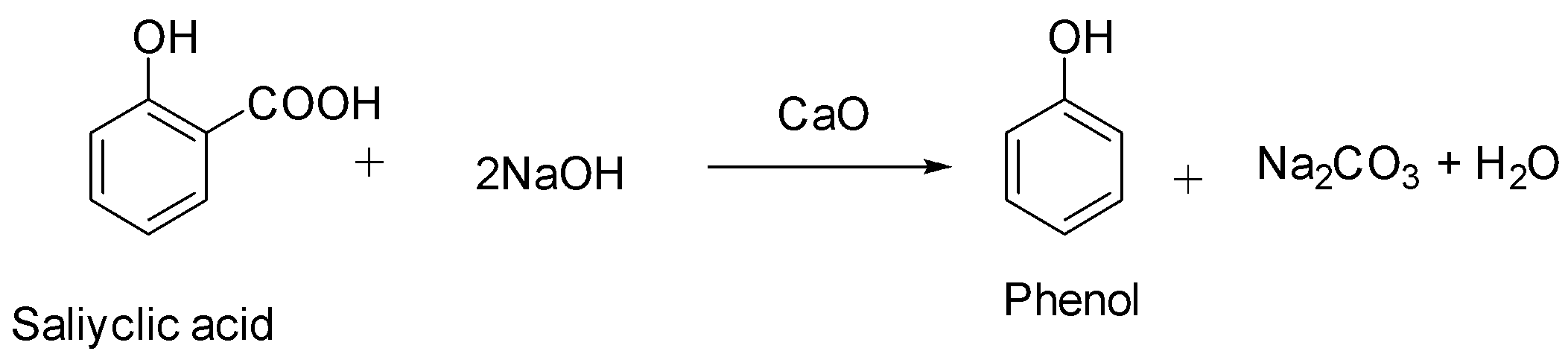
Hence, the correct option among the following is option A (salicylic acid).
Additional Information:-
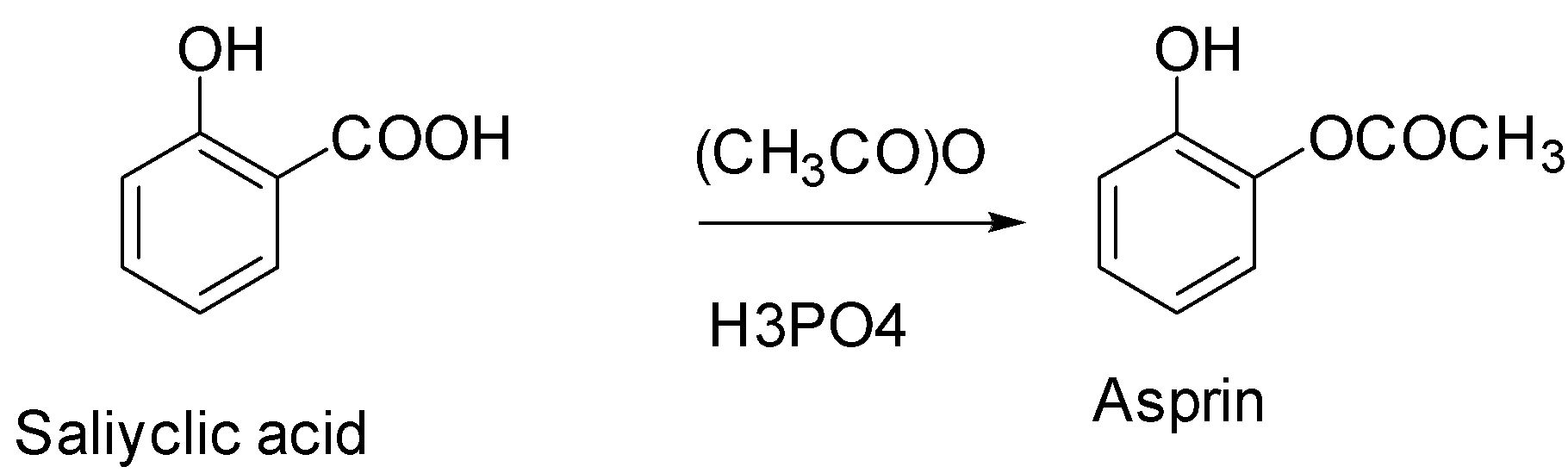
(1) Salicylic acid is a very important precursor for the preparation of aspirin. Aspirin is a drug given to a patient shortly after a heart attack as it decreases the risk of death.
(2) We can prepare aspirin by treating salicylic acid with acetic acid in the presence of an acid catalyst. The phenol group on the salicylic acid forms an ester with the carboxyl group on the acetic acid.
Note:
(1) Decarboxylation is a chemical process that involves the removal of carboxyl groups and releases carbon dioxide from the reaction system. Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain.
(2) Decarboxylation of sodium salts of carboxylic acids by using soda lime to form alkanes is known as the Duma reaction.
(3) The mixture of calcium hydroxide and sodium or potassium hydroxide (soda lime), both are corrosive materials. It is corrosive to metals and tissue. Hence, should be handled carefully.
