Question
Question: Which of the following will best convert nitrobenzene into 3-fluorobromobenzene? 
A. AlCl3F2, Zn/HCl , HClNaNo2−0∘C , CuBr
B. FeBr3Br2 ,HClSnCl2. , HBF4NaNo2−0∘C , heat
C. HClSnCl2 , HBF4NaNo2−0∘C , heat,
FeBr3Br2
D. HClSnCl2 , FeBr3Br2 , HBF4NaNo2−0∘C , heat
Solution
Nitrobenzene is brominated to form 1-bromo-3-nitrobenzene. And then the Nitro group is reduced to an amino group to obtain 3-bromoaniline. The amino group is diazotized and further replaced by Fluorine (F) to obtain 3-fluorobromobenzene.
Complete step by step answer:
Nitrobenzene is an organic compound with the chemical formula C6H5NO2 . It is a water insoluble pale-yellow oil with an almond-like odour. The best way to convert nitrobenzene into 3-fluorobromobenzene is:
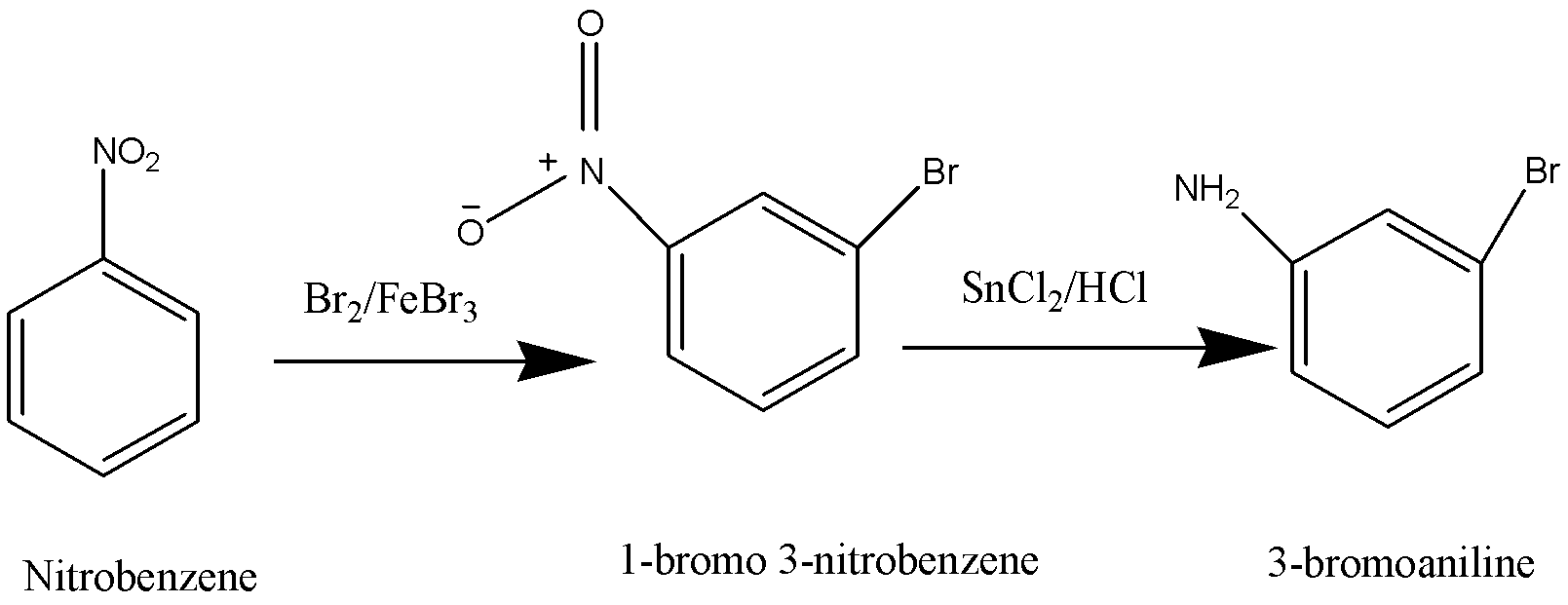
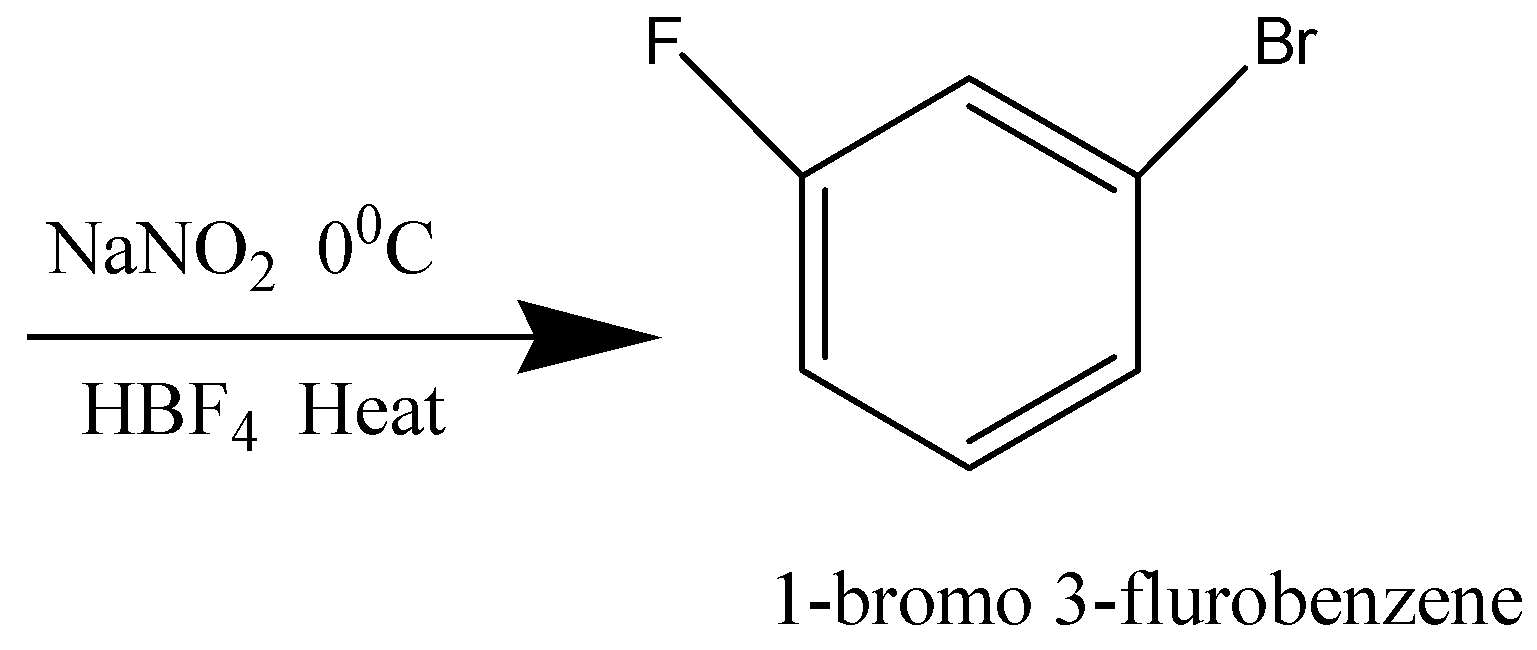
Nitrobenzene is brominated to form 1-bromo-3-nitrobenzene. Nitro group is then reduced to amino group to obtain 3 – bromoaniline. The amino group is then diazotized and it is then replaced by a Fluorine (F) so that we obtain 1-bromo-3-fluorobenzene or 3-fluorobromobenzene. Thus, the reagents which is given in the option B are used to make 3-
fluorobromobenzene.
Therefore, the correct option is (B).
Note: 3-fluorobromobenzene is a clear colourless light yellow. The molecular formula of 3-fluorobromobenzene or 1-bromo-3-fluorobenzene is C6H4BrF . Its molecular weight is 175 g / mol. Its melting point is −8∘C and its boiling point is 149−151∘C (lit). Its flash point is 102∘F . Its density is 1.567 g / mL at 25∘C (lit). It is insoluble in water. Its solubility is 0.4 g / l. It is liquid in form. It is used as an Intermediate of Liquid Crystals.
