Question
Question: Which of the following will be able to show geometrical isomerism? A \(M{{A}_{3}}B\) Square planar...
Which of the following will be able to show geometrical isomerism?
A MA3B Square planar
B MA2B2 Tetrahedral
C MABCD Square planar
D MABCD Tetrahedral
Solution
Isomers in chemistry are molecules or polyatomic ions that have the same molecular formula — that is, the same number of atoms of each element — but different atomic configurations in space. Isomerism refers to the existence or potential of isomers.
Complete answer:
Isomers don't always have the same chemical or physical characteristics as one another. Structural or constitutional isomerism, in which the bonds between the atoms differ, and stereoisomerism or spatial isomerism, in which the bonds are the same but the relative locations of the atoms differ, are the two primary types of isomerism.
Cis–trans isomerism is a word used in organic chemistry. It is also known as geometric isomerism or configurational isomerism. "Cis" and "trans" are Latin prefixes that mean "this side of" and "the other side of," respectively. In chemistry, cis means that the functional groups (substituents) are on the same side of a plane, whereas trans means that they are on the other side. Stereoisomers are pairs of molecules with the same formula but distinct functional groups in three-dimensional space. Cis-trans isomers are stereoisomers. E–Z isomerism, which is an absolute stereochemical description, does not necessarily match to cis-trans notation.
Only the square planar configuration will display geometrical isomerism out of the tetrahedral and square planar layouts.
All of the configurations in MA3B will be identical, therefore there will be no geometrical isomerism. As a result, geometrical isomerism will only be demonstrated by MABCD.
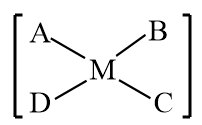
Hence option c is correct.
Note:
The stereochemistry (spatial arrangement of atoms) chosen by certain chemical compounds is described by square planar molecular geometry in chemistry. The atoms of molecules with this shape are positioned in the corners, as the name implies. This shape is used by a large number of compounds, with transition metal complexes providing the most instances.
