Question
Question: Which of the following, upon treatment with tert-BuONa followed by addition of bromine water, fails ...
Which of the following, upon treatment with tert-BuONa followed by addition of bromine water, fails to decolourise the colour of bromine?
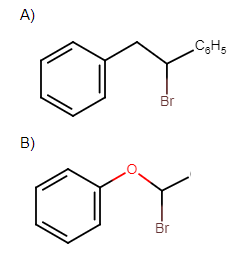
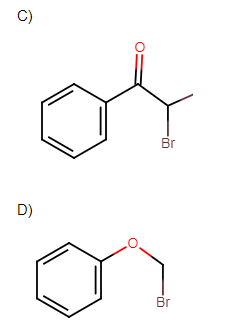
Solution
We know that potassium tert-butoxide is a non-nucleophilic base. It is also very bulky in size. Due to which it helps in the elimination reaction. The mechanism involves the E-2 mechanism.
Complete step-by-step answer:
It is known to us that the nucleophile can act as a base and abstract acidic hydrogen and nucleophilic substitution reactions will take place. This depends upon the strength of the nucleophile. If the nucleophile is poor, then elimination will take place, on the other hand, if it is strong in nucleophilic strength then its substitution reaction will take place.
All other molecules except in option D contain double bonds and a leaving group is in the alpha position. So, elimination reactions will take place. While in only option D no elimination reaction will take place.
In option d, reaction takes place as –
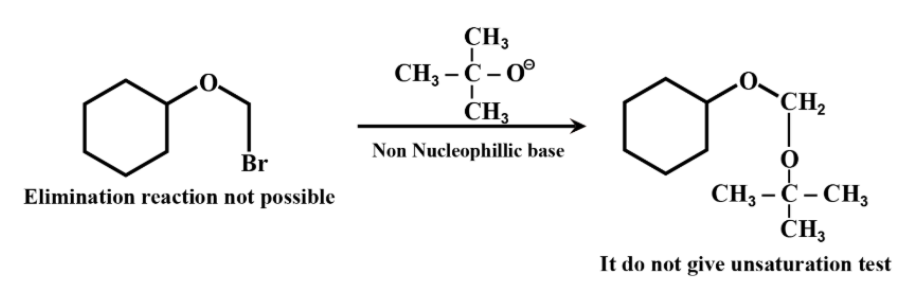
Hence the correct answer is option ‘D’.
Note: Thus, the role of nucleophile as elimination and substitution also depends upon its size. If it is bulky in nature then it acts as a base. And if it is small in size, then substitution will take place.
