Question
Question: Which of the following undergoes nucleophilic substitution by \({S_N}1\) mechanism at fastest rate: ...
Which of the following undergoes nucleophilic substitution by SN1 mechanism at fastest rate:
A. Ethyl chloride
B.Isopropyl chloride
C.Benzyl chloride
D.Chloro benzene
Solution
As we know that SN1 is an unimolecular reaction. In SN1 reaction the intermediate formed is a carbocation. We will have to analyse the stability of the carbocation formed to get an idea about the rate of the reaction.
Complete step by step answer:
We know that SN1 reaction has carbocation as the intermediate. The compound with most stable carbocation will undergo the fastest SN1 mechanism. The electron donating groups stabilize the carbocation. That means resonance, inductive effect and hyperconjugation makes the carbocation stable. Ethyl chloride, isopropyl chloride and chloro benzene cannot undergo resonance . But in benzyl chloride , when the chlorine leaves via nucleophilic substitution by SN1 mechanism , the carbocation formed stabilises because it undergoes resonance with the benzene ring which act as an electron group. So it automatically stabilizes the carbocation. The most stable carbocation will undergo the fastest nucleophilic substitution by SN1 mechanism.
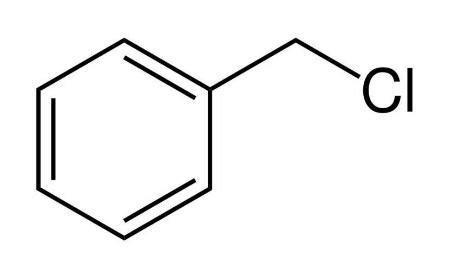
So from the above explanation it is clear to us that the correct option of the given question is C Benzyl chloride.
Additional information:
The order of SN1 mechanism is 3∘>2∘>1∘ , that is tertiary >secondary> primary. SN1 mechanism is preferred in polar protic solvent. SN2 mechanism is preferred in polar aprotic solvent.
Note:
Always remember that in SN1 mechanism we check the stability of the carbocation formed. The electron donating groups stabilize the carbocation thus it decides the rate of the SN1 mechanism. SN1 mechanism is preferred in polar protic solvent. Resonance stabilizes the carbocation formed in the nucleophilic substitution by SN1 mechanism in the benzyl chloride.
