Question
Question: Which of the following undergoes nucleophilic substitution exclusively by \( {{\text{S}}_{\text{N}}}...
Which of the following undergoes nucleophilic substitution exclusively by SN2 mechanism?
(A) Ethyl chloride
(B) Isopropyl chloride
(C) Chlorobenzene
(D) Benzyl chloride
Solution
Hint : There are two types of nucleophilic substitution reactions:- SN1 and SN2 . In SN2 reaction, the approach of the nucleophile towards an alkyl halide and the departure of the leaving group take place simultaneously in a single step.
The overall order of reactivity of alkyl halides in SN2 reactions is – methyl alkyl halides > primary alkyl halides > secondary alkyl halides > tertiary alkyl halides.
Complete Step By Step Answer:
When a carbon atom is attached to only one other carbon atom, it is called a primary carbon atom. When a carbon atom is attached to other carbon atoms on two different sides, it is a secondary carbon and if attached to other carbons on three different sides, it is called a tertiary carbon. If in an alkyl halide, the halogen is bonded to a primary, secondary and tertiary carbon, then it is called a primary, secondary and tertiary alkyl halide respectively.
The first option is ethyl chloride. It is a primary alkyl halide. Since primary alkyl halides prefer to undergo substitution by SN2 mechanism where the nucleophile always attacks from the backside, ethyl chloride will undergo nucleophilic substitution exclusively by SN2 mechanism.
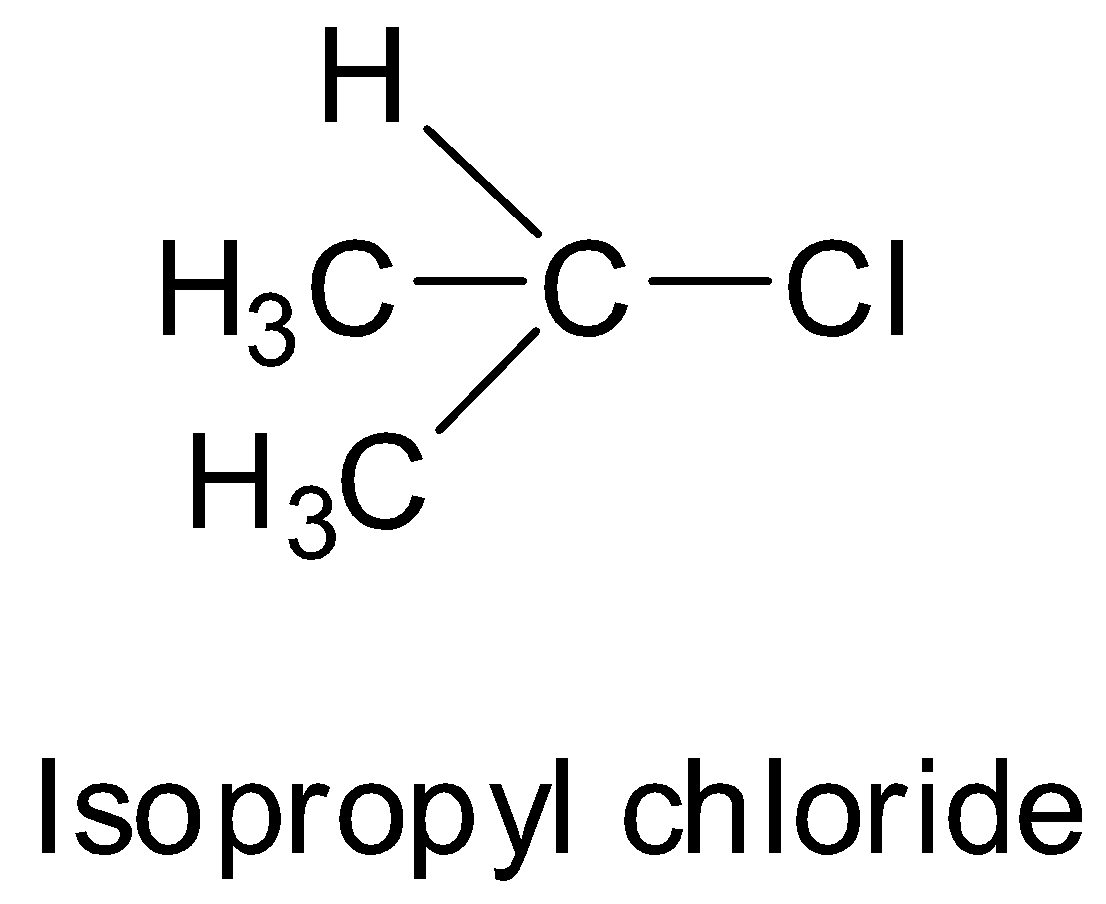
Isopropyl chloride is a secondary alkyl halide. So, although it can undergo SN2 substitution, its tendency will be less than ethyl chloride which is a primary alkyl halide because secondary alkyl halides are bulkier than primary alkyl halides and this makes the approach of the nucleophile difficult.
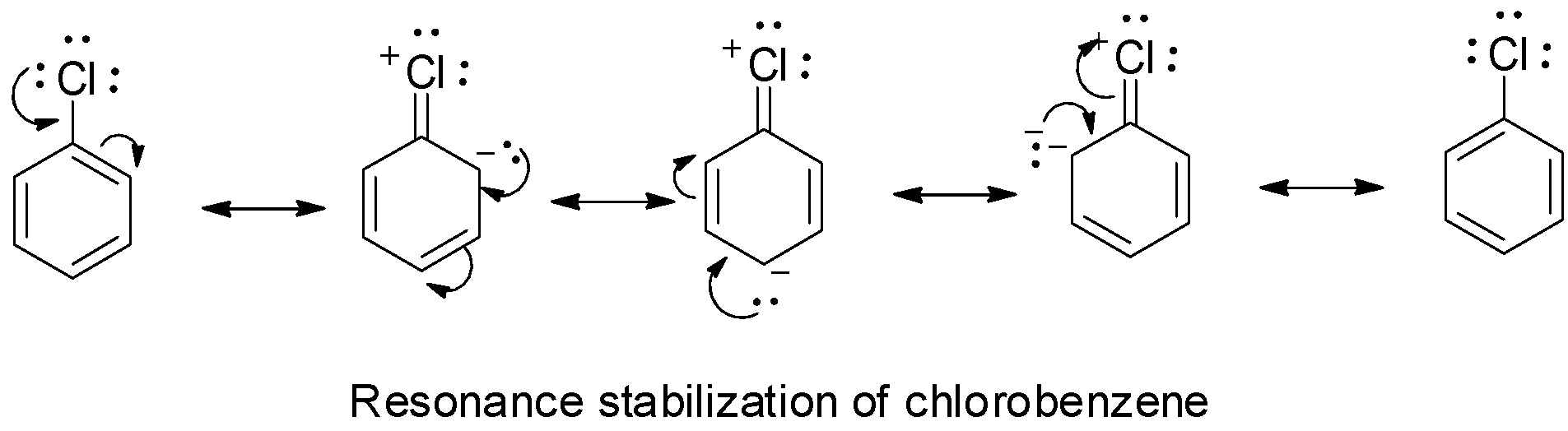
Chlorobenzene is an aryl halide and aryl halides are less reactive in comparison to alkyl halides towards either SN1 or SN2 nucleophilic substitution as the carbon – halogen bond in aryl halides is strong due to stabilization by resonance and hence cannot be easily broken.
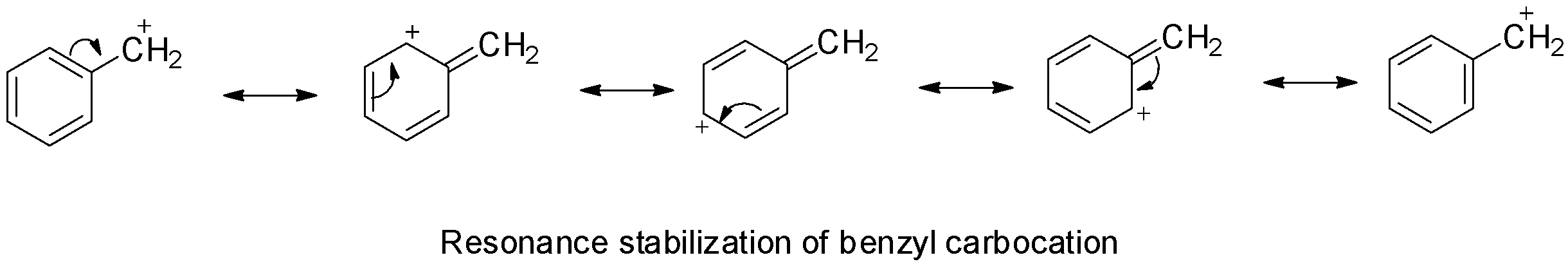
Benzyl chloride is a primary benzylic halide and due to stabilization of benzyl carbocation by resonance, primary benzylic halides show higher reactivity towards SN1 than SN2 substitution.
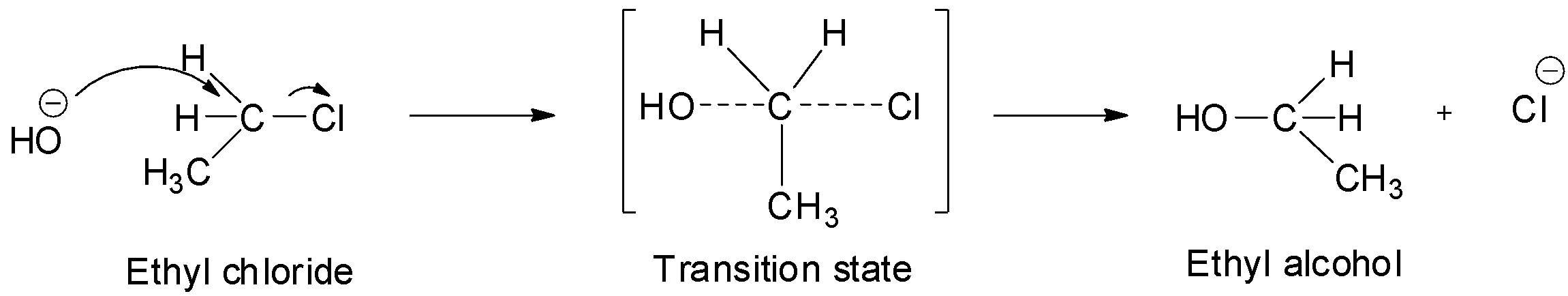
Thus, only ethyl chloride will exclusively undergo SN2 substitution.
So, option A is correct.
Note :
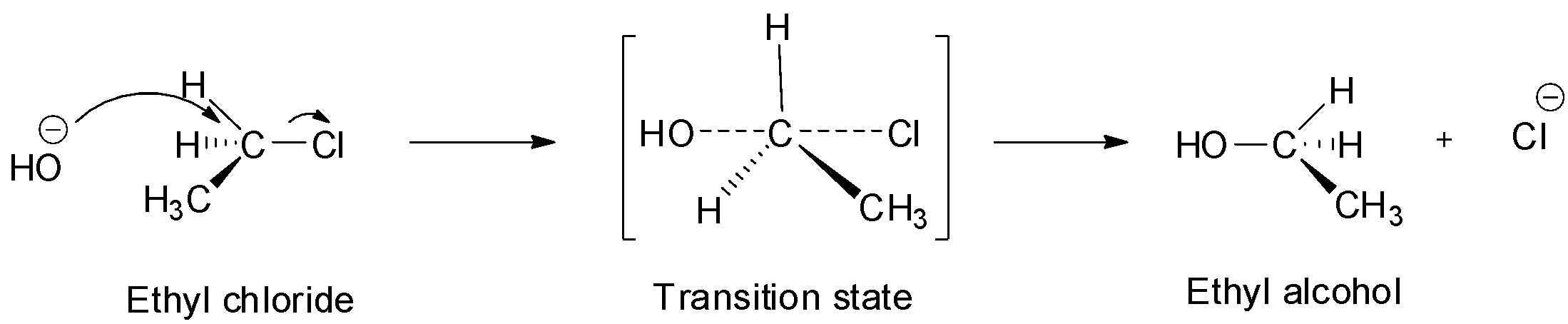
In SN2 substitution reactions, the attack of the nucleophile is from the opposite side to the alpha carbon carrying the halogen. The carbon-halogen bond starts breaking and a new carbon-hydroxyl bond starts forming. These two processes take place simultaneously in a single step and there is no formation of intermediate. Thus, this reaction occurs via a transition state in which both the reactants are partially bonded to each other. The carbon atom in the transition state is simultaneously bonded to both the incoming nucleophile and outgoing leaving group which means it is bonded to 5 atoms which make it unstable and it ultimately decomposes to give the final ethyl alcohol product. Since in SN2 substitution reactions, the nucleophile attacks from the back side and the leaving group leaves from front side, they are always accompanied by inversion of configuration. This inversion of configuration is known as Walden inversion.
