Question
Question: Which of the following treatments with \( NaN{{H}_{2}} \) in liquid \( N{{H}_{3}} \) gives m-anisidi...
Which of the following treatments with NaNH2 in liquid NH3 gives m-anisidine?
(A) o-aminoanisole.
(B) m-aminoanisole.
(C) Both A and B
(D) p-aminoanisole.
Solution
Hint : We know that the benzene mechanism is followed in this reaction. It is formed as benzene then further reaction occurs. Ortho, para bromoanisole is an electron withdrawing group, it undergoes electrophilic substitution. The halogenation will take place only at the ortho and para positions for anisole and not at the Meta position
Complete Step By Step Answer:
First, think about the chemical formula and structure of anisole. Anisole is a mono methoxy benzene that is benzene substituted by a methoxy group. It has a role as a plant metabolite. It is an ether. The terms anisole and aniline are different from each other. Aniline is the simplest aromatic amine in which the benzene ring is attached to the amino group whereas anisole is an ether in which the benzene ring is attached to the methoxy group.
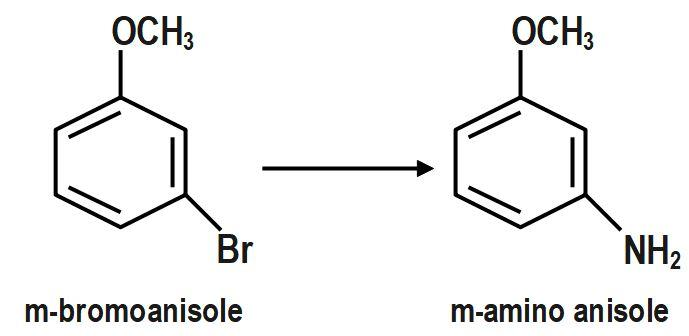
The product is a mixture of para- and meta-methoxyaniline. Here, we get two products para- and meta-methoxyaniline. Among these two, as we can see para-bromoanisole is the major product and the yield is around 90 for it and the ortho-bromoanisole is the minor product with a very low yield. This is due to the steric hindrance in the ortho position as a bulky methoxy group is attached to the benzene ring.
Therefore, the correct answer is option B.
Additional Information:
It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. It is a precursor to other synthetic compounds. Anisole undergoes electrophilic aromatic substitution reaction more quickly than benzene does, when in turn reacts more quickly than nitrobenzene. The methoxy group is an ortho/para directing group, which means that the electrophilic substitution reaction preferentially occurs at these three sites. The enhanced nucleophilicity of anisole vs benzene reflects the influence of the methoxy group, which renders the ring more electron-rich. Anisole is a precursor to perfumes, insect pheromones and pharmaceuticals. Its main hazard is its flammability.
Note :
Note that as we can see in the above discussion we mentioned that electron withdrawing groups increase the electron density at ortho and para position but the reason behind it can be understood from the resonance hybrid structures of anisole. This is a nucleophilic reaction following the benzene mechanism.
