Question
Question: Which of the following structures is a ring chain isomer of each other? A) 
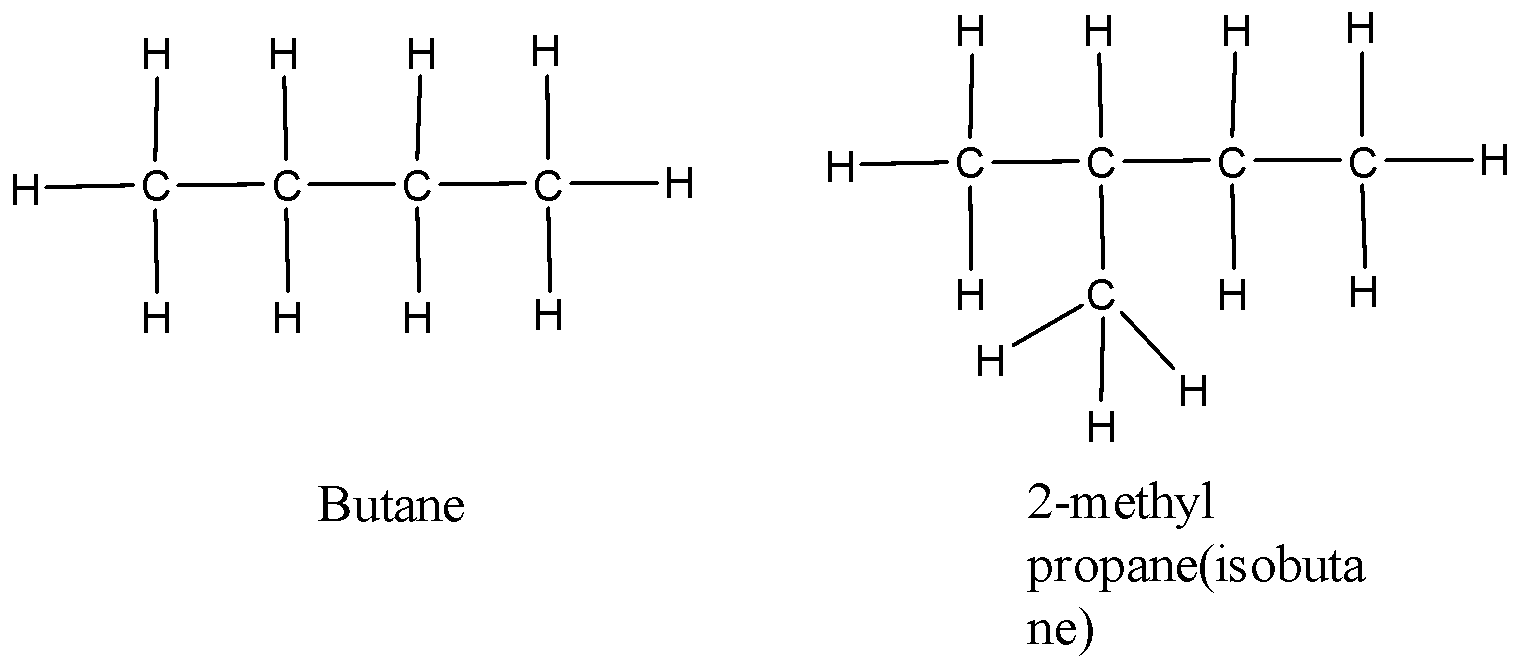
B)
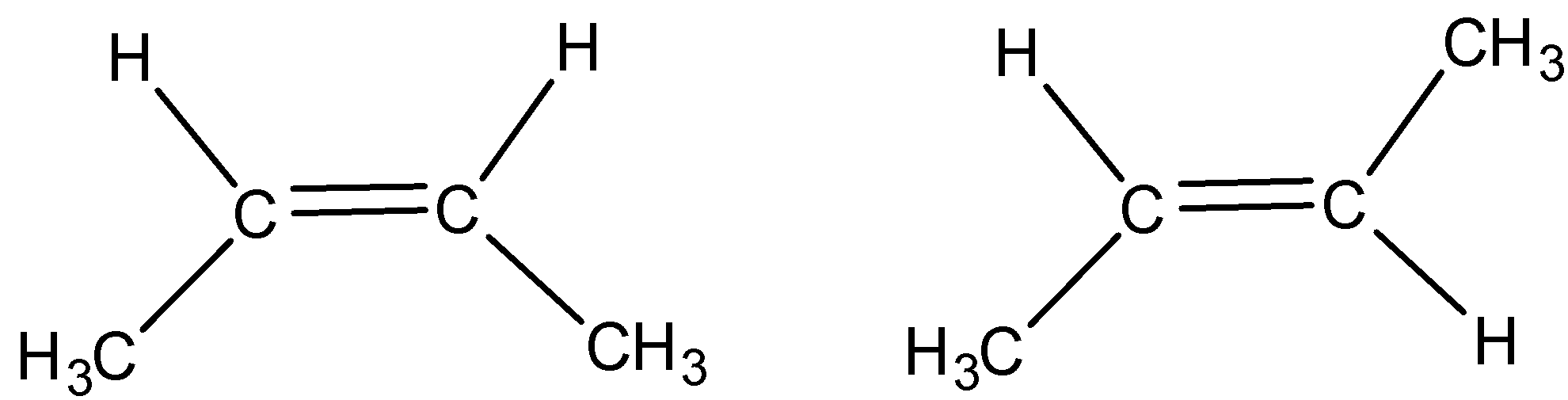
C)
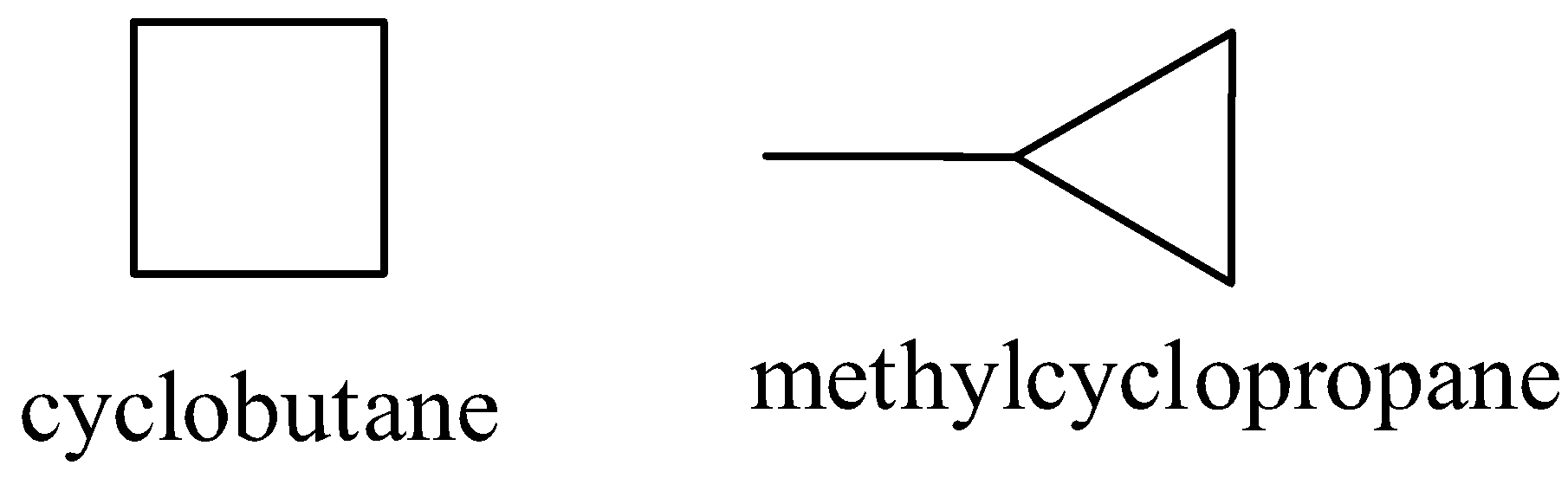
D)
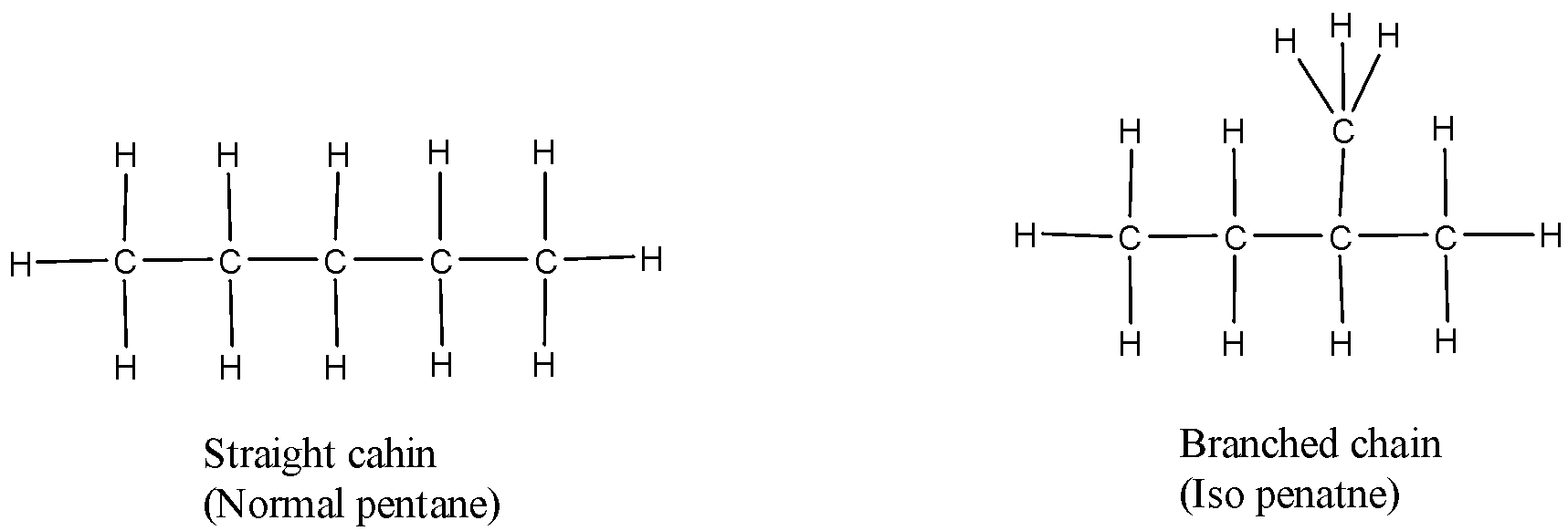
Solution
We know that the molecules which have the same molecular formula but different molecular geometries are called isomers. There are several types of isomers. They are,
Conformational isomers.
Constitutional isomers.
Stereoisomers.
Complete step by step answer:
First, we have to know about structural isomers.
Structural isomer:
The molecules which have the same molecular formula but differ by the atoms or bonds are called a structural isomer. It is also known as a constitutional isomer. It is divided into two types (1) chain isomers and (2) ring isomers.
Consider the molecules in option A and D. The molecular formula of both the compounds in option A is C4H10 but butane is linear while isobutane is branched. Thus option A is a chain isomer, not a ring isomer. Likewise, the molecular formula of both the compounds in option D is C5H12 but pentane is linear while isopentane is branched. Thus option D is a Chain isomer, not a ring isomer
Therefore, Option (A) and (B) is incorrect.
Ring isomer:
The isomers which are having the same molecular formula but they have a different structural formula to the type of ring are called ring isomers.
Now, consider the option (C). One of the isomers has a four-membered ring while the other has a three-membered ring. Thus the correct answer is an option (C).
Let us see stereoisomers.
Stereoisomers:
The isomers which differ by the orientation of atoms in space are called stereoisomers. Stereoisomers are of two types: (1) Geometrical isomer and (2) optical isomer.
Now, consider the option D. The molecule 2-butene is a geometrical isomer in which atoms are differently oriented in space. The double bond in 2-butene is not free to rotate and give cis-trans isomers.
Thus an option D is incorrect.
Note:
We must know that the conformational isomers are the isomers that are differing by their rotation around a single bond.
In cis isomer, the same type of atoms presents on the same side while in Tran’s isomers the same type of atoms presents on the opposite side.
