Question
Question: Which of the following structures cannot represent resonance form for diamagnetic \({N_2}O\)? A) !...
Which of the following structures cannot represent resonance form for diamagnetic N2O?
A) 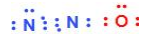
B) 
C) 
D) 
Solution
To find the structure of a certain molecule, we must recall the electronic configurations of the constituent atoms of the molecule. Valence electrons of an atom are used to form bonds and determine the structure of the molecule. Nitrogen has 5 valence electrons and oxygen has 6 valence electrons.
Complete step by step solution:
In nitrous oxide, the central atom is a nitrogen atom which is bonded to an oxygen atom on one side and to a nitrogen on the other. Resonance involves only the delocalization of electrons. The atoms do not change positions, only the bonding between the atoms changes. Of the given structures, we can see clearly that A and B represent nitrous oxide.
In option C and D, the central atom is given as oxygen which is not the correct representation for nitrous oxide as the position of atoms has been altered.
In option D, the given compound has three nitrogen atoms and no oxygen. Thus it clearly does not represent nitrous oxide.
So, the answers are options C, D and E.
Additional information:
Among nitrogen and oxygen, we know that oxygen is more electronegative than nitrogen. It is preferred that the less electronegative element nitrogen does not carry a negative charge and that resonance structure is more stable and hence more contributing. If we compare option A and B, the structure in option A is more stable resonance structure as in B, the less electronegative atom nitrogen carries a negative charge
Note:
Resonance structures of a molecule describe the delocalization of electrons. They are a set of Lewis structures and are also known as canonical structures.
