Question
Question: Which of the following structure represents the transition state of slow step of reaction I. ...
Which of the following structure represents the transition state of slow step of reaction I.
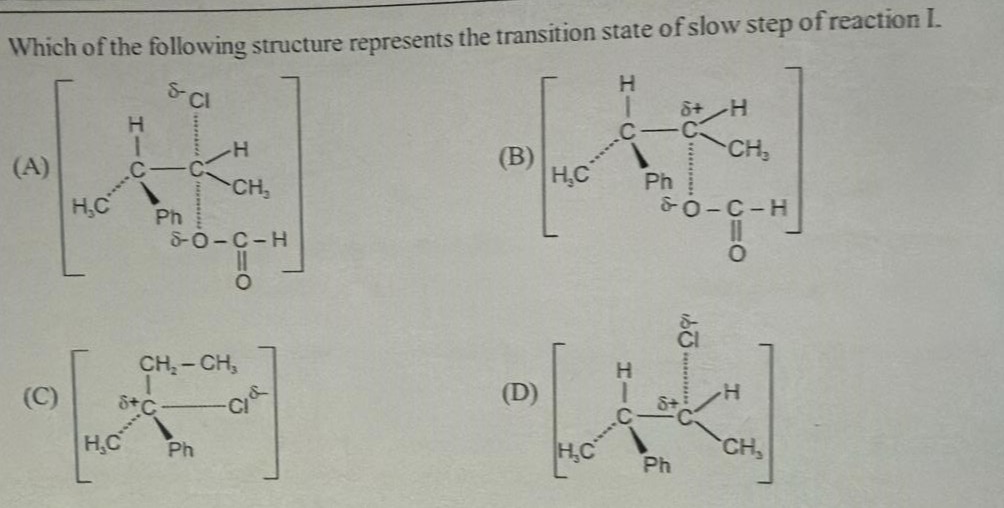
(A)
(B)
(C)
(D)
D
Solution
The question asks to identify the transition state of the slow step of reaction I from the given options. Transition states are high-energy, unstable structures that represent the point of maximum energy along the reaction coordinate. They are typically depicted with partial bonds (dotted lines) and partial charges (δ+ and δ−).
Let's analyze each option:
(A) This structure represents a transition state involving two carbon atoms, a chlorine atom, and a carboxylate-like group. It shows partial bonds between the two carbon atoms, and partial bonds between one carbon and Cl, and the other carbon and the oxygen of the carboxylate group. This could potentially be a transition state for a reaction involving bond formation and breaking, but it is not a standard representation of a common reaction's slow step.
(B) This structure shows a transition state involving a central carbon, a hydrogen atom, and a carboxylate-like group. It shows partial positive charge on the central carbon and partial negative charge on the oxygen of the carboxylate group. There are dotted lines to the hydrogen and the oxygen. This might represent a transition state for an elimination reaction or a substitution reaction.
(C) This structure shows a central carbon atom with substituents CH2-CH3, CH3, and Ph, and a dotted line to a chlorine atom. There is a partial positive charge on the carbon and a partial negative charge on the chlorine. This represents the transition state for the ionization of a substrate (CH2-CH3)(CH3)(Ph)C-Cl, leading to the formation of a carbocation and a chloride ion. This is the slow step of an SN1 or E1 reaction.
(D) This structure shows a central carbon atom with substituents H, CH3, and Ph, and a dotted line to a chlorine atom. There is a partial positive charge on the carbon and a partial negative charge on the chlorine. This represents the transition state for the ionization of a substrate (H)(CH3)(Ph)C-Cl, leading to the formation of a carbocation and a chloride ion. This is the slow step of an SN1 or E1 reaction.
Considering the common organic reactions, the slow step of an SN1 or E1 reaction is the ionization of the alkyl halide to form a carbocation and a halide ion. The transition state for this step involves the partial breaking of the carbon-halogen bond and the development of partial positive charge on the carbon and partial negative charge on the halogen. Both structures (C) and (D) represent such transition states. However, the substituents on the central carbon in (C) are different from those in (D). Without knowing the exact reactant for reaction I, we need to choose the most likely option. Given that options (A), (B), and (D) seem to involve the same core structure around the central carbon (H, CH3, Ph), it is likely that reaction I starts with a substrate containing this fragment. Structure (D) shows the transition state for the ionization of (H)(CH3)(Ph)C-Cl. This is a standard representation of the slow step of an SN1 or E1 reaction.
Let's assume reaction I is the SN1 or E1 reaction of (H)(CH3)(Ph)C-Cl. The slow step is the ionization:
(H)(CH3)(Ph)C-Cl → [(H)(CH3)(Ph)C]δ+ --- [Cl]δ− transition state → [(H)(CH3)(Ph)C]+ + Cl−
The transition state for this step is represented by structure (D).
