Question
Question: Which of the following statements regarding the structure of \(SOC{l_2}\) is not correct ? ( A ) T...
Which of the following statements regarding the structure of SOCl2 is not correct ?
( A ) The sulphur is sp3 hybridised and it has a tetrahedral shape .
( B ) The sulphur is sp3 hybridised and it has a trigonal pyramid shape .
( C ) The oxygen – sulphur bond is pπ – dπ bond .
( D ) It contains one lone pair of electrons in the sp3 hybrid orbital of sulphur .
Solution
As three atoms are bonded to the sulphur atom, we can say that the sulphur atom is sp3 hybridized.And by referring to the shapes of atoms of different hybridization, it’s a trigonal pyramid shape. The shape and geometry of the molecule are defined by minimum repulsion.
For 3 electron pairs, the shape is trigonal pyramidal.
Complete step by step solution:
First, we consider, option ( A )
The SOCl2 molecule is sp3 hybridized.
The formula for finding hybridization of molecules is
Hybridization =21(V+H−C+A)
Here, V = VALENCE ELECTRONS IN CENTRAL METAL ATOM
H = Number of monovalent atom attached to the central metal atom
C = Cation charge
A = Anion charge
Substituting the values for SOCl2,
⇒Hybridization = 21( 6 +2 +0 +0 )
= 21( 8 )
=4
Hence, the hybridization of SOCl2is sp3
Now, the structure of SOCl2 is a trigonal planar because of the electron densities of 2 chlorine atoms and 1 oxygen atom, as given in the image.
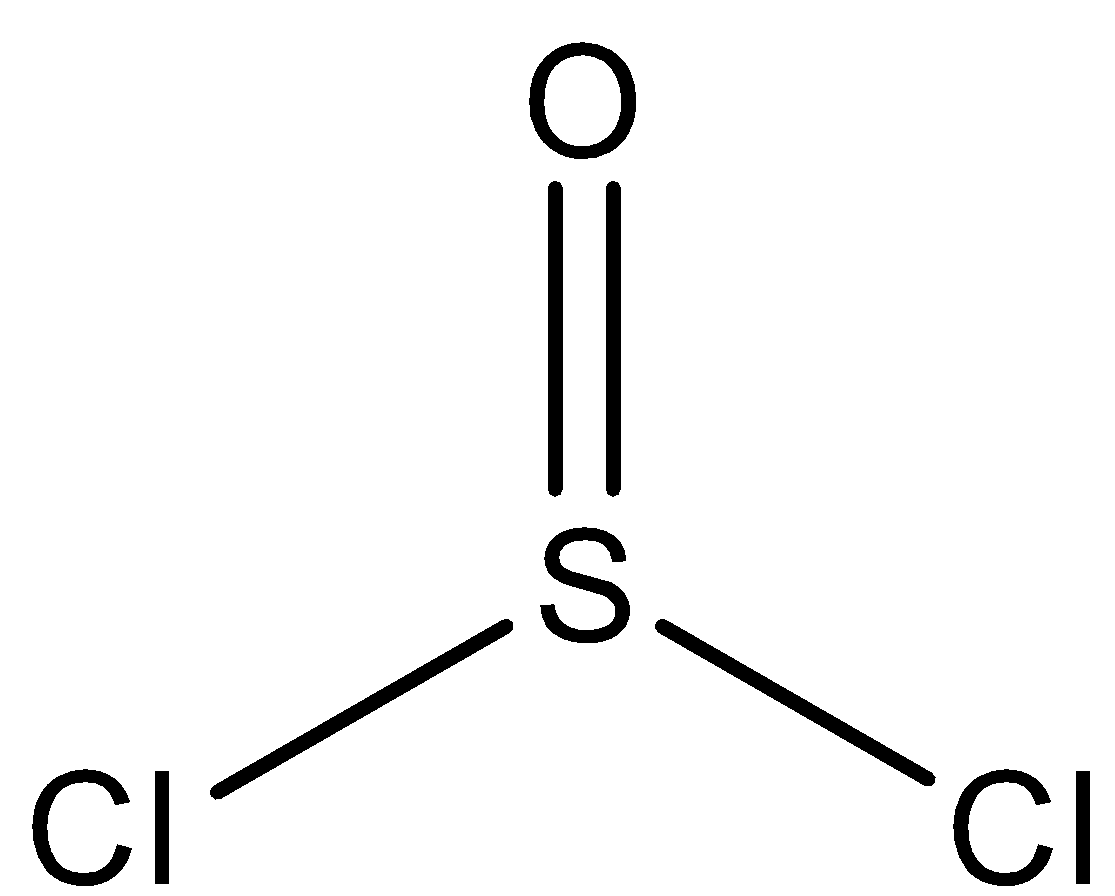
Hence, option ( A ) is incorrect
For option ( B ),
The statement is true, as proved above that the sulphur is sp3 hybridized and it has a trigonal pyramid shape.
Hence, option ( B ) is correct
For option ( C ),
The lone pair of p orbital back bonds with that of d orbital of central atom sulphur.
Hence, option ( C ) is correct
For option ( D ),
The electronic configuration of the sulphur atom is
1s2 2s2 2p6 3s2 3p4
The 4 valence electrons of sulphur bond with 2 chlorine atoms and 1 oxygen atom leaving behind 2 valence electrons out of 6
Hence, the sulphur atom contains 1 lone pair of electrons
Hence, option ( D ) is correct
So, The correct option is ( A ) - The sulphur is sp3 hybridized and it has a tetrahedral shape.
Note: The geometry of SOCl2 is due to the lone pair effect. The VSEPR Theory ( Valence Shell Electron Pair Repulsion ) gives the geometry and shape of molecules.
According to the VSEPR Theory the bond angle between oxygen, sulphur and chlorine are 106∘and between chlorine, sulphur, chlorine is 96∘ respectively.
