Question
Question: Which of the following statements is true about resonance? The resonance hybrid explains all featu...
Which of the following statements is true about resonance?
The resonance hybrid explains all features of a molecule.
A.True
B.False
Solution
A resonance hybrid is an intermediate structure of all the contributing structures of a molecule. It tells about the bond characteristics of the molecule as a whole.
Complete step by step answer:
The representation of resonance in benzene is shown above. The equivalent structure shows the delocalization of electrons on the entire ring and is called the resonance hybrid.
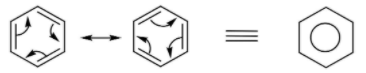
A resonance hybrid represents the delocalization of electrons within the entire molecule.
Of all the resonating structures, the most stable structure contributes the most to the resonance hybrid. The most stable contributing structure has the most number of atoms with complete octets, most number of covalent bonds and least number of formal charges.
The resonance hybrid can only explain some of the bonding features of a molecule and the entire bonding of the molecule cannot be described.
Thus, the given statement is false.
Note:
In a resonance hybrid, all the structures should have the same total number of electrons and the hybridization of the molecule must remain the same. The position of atoms remains unchanged in all the resonating structures, also the number of lone pairs also remains the same. The most stable structure has least separation of formal charge, the most electronegative atom bears the negative charge and the most electropositive atom bears the positive charge.
