Question
Question: Which of the following statements is true about ethene. A. Both carbon atoms are \({\text{s}}{{\te...
Which of the following statements is true about ethene.
A. Both carbon atoms are sp2 hybridized and the molecule is planar.
B. Both carbon atoms are sp2 and all bond angles are approximately 109.5∘
C. One carbon atom is sp hybridized and the other is sp2
D. Both carbon atoms are sp3 hybridized and with bond angles are approximately 109.5∘
E. Both carbon atoms are sp hybridized and the molecule is planar.
Solution
In this question, we are using the concept of finding the hybridisation of the both carbon atoms of the ethene molecule and then trying to understand its shape.
Complete step by step answer:
As we know that the ethene is a hydrocarbon with the chemical formula …. It is the simplest alkene. It has a faint “ sweet and musky” odour when it is present in the pure form. It is a flammable gas. It has a molecular mass of 28u.Ethene is represented as
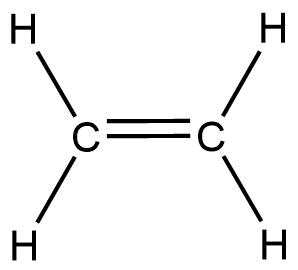
Ethene molecule contains 5 sigma and 1 pi-bond For the formation of the molecule, the ground state electronic configuration is 1s22s22p2
During the formation of C2H4, the ground state electronic configuration of carbon atom gets excited and changes to 1s22s22px12py12pz1 One electron jumps from 2s orbital to 2p because it has to form bonds with the other carbon atoms and hydrogen atom. In this way, we have four unpaired electrons in the excited stage of carbon. Meanwhile, out of 2px,2py,2pz and 2sorbitals, only 2s,2px,2py orbitals takes part in the hybridization which leads to the formation of sp2 hybridized orbital. The hybridized orbitals form a ∏ bond with other carbon atoms. Similarly, is the case for other carbon atoms. So, we found that each carbon atom of the ethene molecule is sp2 hybridized. C2H4 geometry is said to be planar in structure. Hence, the option (A) is correct which states that both carbon atoms are sp2 hybridized and the molecule of ethene is planar in nature.
Hence option (A) is the correct answer.
Note:
In ethene, each carbon atom combines with three other atoms whereas in ethane the carbon atom is combined with the four other atoms.The simplest organic compounds are the alkanes. Alkanes have only single bonds between carbon atoms and are called saturated hydrocarbons. Alkenes have at least one carbon-carbon double bond.Alkenes and alkynes are called unsaturated hydrocarbons.
