Question
Question: Which of the following statements is not correct? (A) bis (glycinatio) zinc (II) is optically acti...
Which of the following statements is not correct?
(A) bis (glycinatio) zinc (II) is optically active.
(B) [NiCl4]2− and [PtCl4]2− have different shapes.
(C) [NiCl4]2− is a square planar complex.
(D) [Ni(CN)4]2− and [Ni(CO)4] have the same magnetic moment.
Solution
In order to find which of the following statements is correct and which is incorrect, we have to discuss each statement separately and see which is incorrect. We have to discuss optical activity, different shapes and magnetic moments respectively.
Complete step by step answer:
Let us discuss each option to see which are not the correct statements.
- First is the option (A). The structure of bis(glycinatio)zinc (II) is given below. On seeing the structure, we can say that bis(glycinatio)zinc (II) will not have any plane of symmetry. Therefore, bis(glycinatio)zinc (II) is optically active.
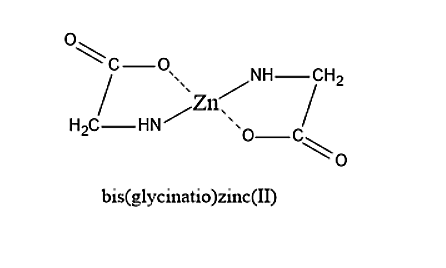
Therefore, the given statement is correct.
- Now let us discuss the option(B). let us see the shape of [NiCl4]2−and [PtCl4]2−.
The nickel is having an atomic number of 28 and therefore the electronic configuration of nickel is [Ar]3d84s2. In [NiCl4]2−,the nickel is having an oxidation state of +2. The electronic configuration of nickel in [NiCl4]2− is [Ar]3d8. Therefore, the hybridisation of [NiCl4]2− is sp3 and it will be having a tetrahedral structure.
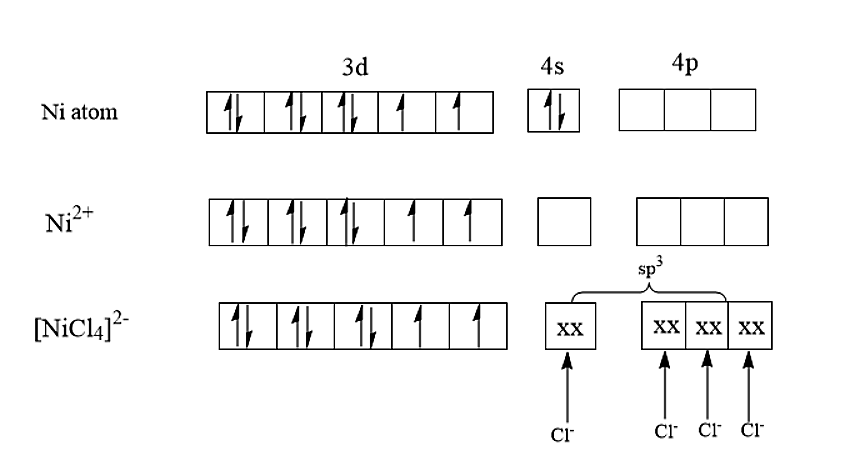
The atomic number of Platinum is 78 and the electronic configuration of Platinum was found to be [Xe]4f145d96s1. In [PtCl4]2−, the platinum is having an electronic configuration of [Xe]4f145d8. Therefore, it is having a hybridisation of dsp2 and therefore, it is having a square planar structure.
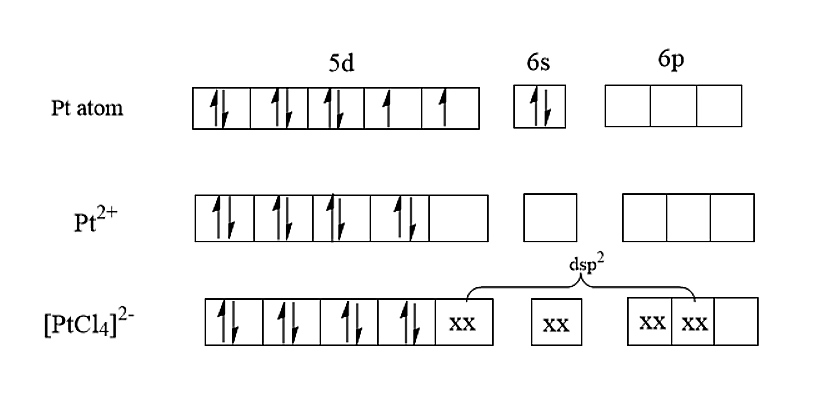
Hence it is proved that both [PtCl4]2− and [NiCl4]2− are having different shapes.
Therefore, the given statement is correct.
- Now let us discuss the option (C). The nickel is having an atomic number of 28 and therefore the electronic configuration of nickel is [Ar]3d84s2. In [NiCl4]2−,the nickel is having an oxidation state of +2. The electronic configuration of nickel in [NiCl4]2− is [Ar]3d8. Therefore, the hybridisation of [NiCl4]2− is sp3 and it will be having a tetrahedral structure.
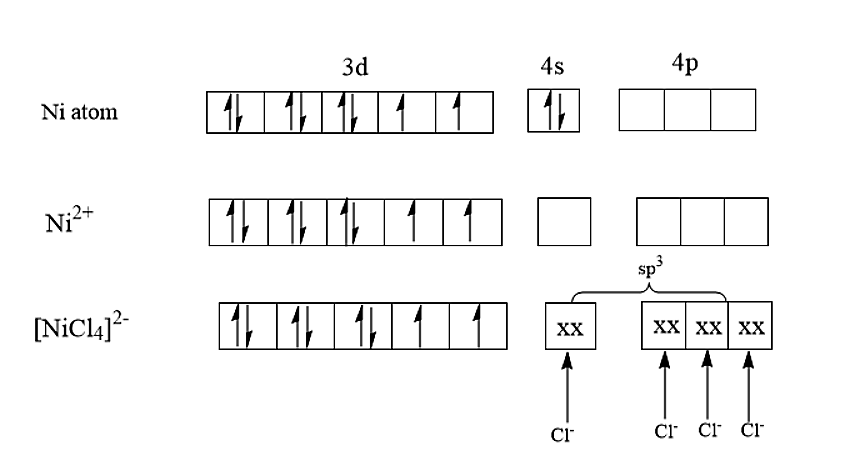
Therefore, the given statement saying [NiCl4]2− is a square planar complex is incorrect.
- Let us now discuss the option (D). [Ni(CN)4]2− is having dsp2 hybridisation and therefore the structure is square planar. [Ni(CO)4] is having sp3 hybridisation and therefore the structure is tetrahedral. From the below image, we can see that all the spins are paired in both the complexes i.e., [Ni(CN)4]2− and [Ni(CO)4]. Both are diamagnetic in nature.
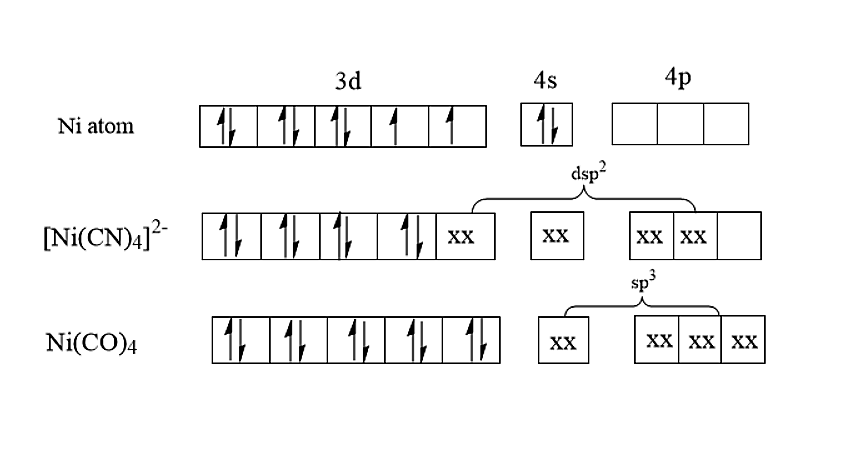
Therefore, the given statement is correct.
So the correct answer is “C”:
Note: We have to remember that the paramagnetic and diamagnetic are different from one another. When the spins in the orbitals are paired, then it is said to be paramagnetic. When the spins in the orbitals are unpaired, then it is called paramagnetic.
