Question
Question: Which of the following statements is incorrect for electrophilic substitutions? (A) Ortho- and par...
Which of the following statements is incorrect for electrophilic substitutions?
(A) Ortho- and para- directing groups increase electron density at ortho- and para- positions.
(B) Meta- directing groups increase electron density at meta- position.
(C) Meta- directing groups decrease electron density at meta- position.
(D) Ortho- and para- directing groups decrease electron density at meta- position.
Solution
A substitution reaction in which the functional group present in a molecule or attached to the molecule is substituted or replaced by an electrophile is termed as ‘electrophilic substitution reaction’.
The position to be occupied by a second substituent as well as the reactivity of the monosubstituted benzene ring is determined by the inductive effect and resonance effect.
Complete step by step solution:
The ability of a group already present on the benzene ring to direct the next incoming group is termed as the directive influence of the group. The group which is already present on the benzene ring plays a key role in deciding about the reactivity of the benzene ring towards further electrophilic substitutions.
These are classified into two classes: ortho and para directing groups and meta directing groups.
Now, generally electron withdrawing groups are deactivating groups and electron donating groups are activating groups.
All ortho and para directing groups have one or more lone pairs of electrons on the atom which is directly attached to the benzene ring. Because of the resonance effect, the ortho and para positions of the ring obtain high electron density as compared to meta position. All such groups are called activating groups.
For example, consider the orientation effect of the amine group in aniline.
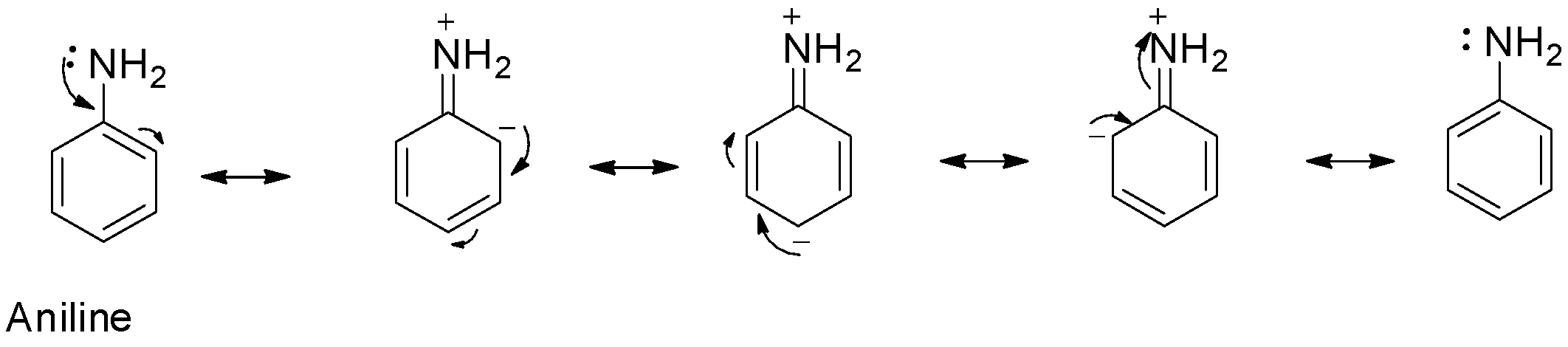
From the above resonating structures, it is seen that increased electron density is at ortho and para positions. Thus, electrophilic substitution will occur at the ortho and para positions. So, option A is correct and D is correct.
In case of meta directing groups like nitro groups, the atom directly attached to the benzene ring is further linked to more electronegative atoms like oxygen by multiple bonds. Thus, they withdraw electrons from all positions of the ring. Due to this, electron density decreases at ortho and para positions and so such groups deactivates the ring towards further substitution.
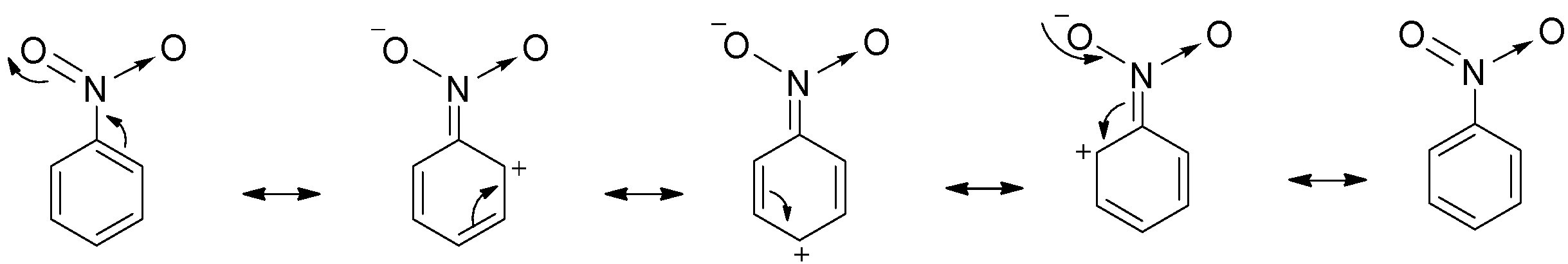
The electron density is relatively more at the meta position and so meta directing groups direct the new group at meta position and so option B is correct and C is incorrect.
Note:
Larger the size of the alkyl group already present, the smaller will be the percentage of the ortho isomer formed in an electrophilic aromatic substitution.
The bulky alkyl groups cause steric hindrance to the introduction of new incoming substituents in the ortho positions.
