Question
Question: Which of the following statements is correct while conduction of electricity in lemon juice? A.The...
Which of the following statements is correct while conduction of electricity in lemon juice?
A.The liquid (lemon juice) allows the electric current to pass through. Hence, the circuit of the tester is complete.
B.The current flows in the circuit of the tester.
C.The bulb glows which indicates lemon juice is a good conductor of electricity.
D.All of the above.
Solution
As we know, lemon juice contains citric acid. Citric acid is a strong electrolyte. This acid breaks up into positive and negative ions when dissolved in water and hence can conduct electricity because the charged particles are able to flow within the acid.
-When rods made of copper and zinc are placed in the lemon juice, a chemical reaction occurs. Within the lemon, electrons flow from the copper rod to the zinc rod, turning the copper and zinc into positive and negative electrodes respectively.
Complete answer:
Citric acid (C6H8O7)is a naturally occurring acid found in lemon juice. It is a weak organic acid. The systematic name of citric acid is 2-hydroxypropane-1,2,3-tricarboxylic acid. If we look at the molecular figure shown in the diagram, we can notice OHs which are sitting next to =O′s. Those are acid groups. We know that acids are substances that can release H+ions (hydrogen ions) when they are dissolved in water.
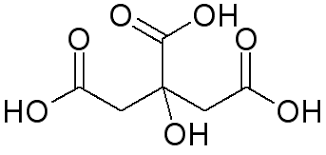 Fig. Molecular structure of Citric acid.
Fig. Molecular structure of Citric acid.
Hence, we can say lemons are acidic in nature.
When dissolved in water, lemon juice can conduct electricity as the acid within it breaks up into anions and cations. The liquid (lemon juice) between the two ends of the tester allows the electric current to pass, and the circuit of the tester becomes complete. Also, the bulb glows which indicates that lemon juice is a good conductor of electricity.
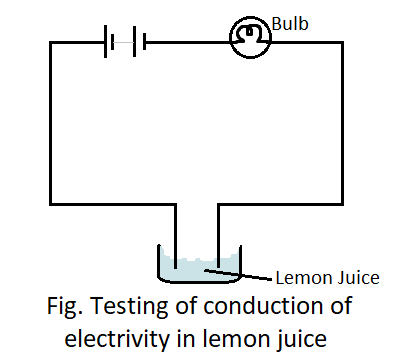
So, The correct answer is (D).
Note: We might expect that citric acid is a strong acid as the three acid groups in citric acid can release (in theory) three H+ ions (hydrogen ions) per molecule. But, it’s not. Chemists call it a weak acid because although it can release three hydrogen ions per molecule, it doesn’t really want that much. It only likes to keep hold of them. The pH of lemon juice is about 2.
