Question
Question: Which of the following statements is correct, about these cycloalkenes? a.) Stability difference b...
Which of the following statements is correct, about these cycloalkenes?
a.) Stability difference between I and II is more than that between III and IV
b.) Stability difference between I and II is less than that between III and IV
c.) Overall stability order is I > II > III > IV
d.) None of these
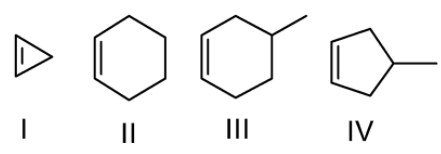
Solution
There are two criteria to decide stability of cycloalkene. One is hyperconjugation and the other is strain of the ring. Hyperconjugation is no bond resonance in which α-hydrogen takes part. Higher the number of α-hydrogens higher will be the number of hyperconjugative structures, higher will be the stability of alkenes. In cycloalkene compounds the ring is planar and that creates strain because the bond angle is less than that actually should have been and that strain makes the ring unstable. Higher the strain, less stable will be the cycloalkene.
Complete step by step answer:
- Cyclopropene(I): In cyclopropene carbons forming double bonds are sp2 hybridized, so bond angle should have been 1200 but ring’s geometry is of equilateral triangle and it means bond angle becomes600.
Strain= 1200−600
Strain= 600
- Cyclohexene(II): In cyclohexane carbons forming double bonds are sp2 hybridized, so bond angle should have been 1200 but ring’s geometry is of regular hexagon and it means bond angle becomes1200 .
Strain= 1200−1200
Strain= 00
- 3-methylCyclohexene(III): In 3-methylcyclohexene carbons forming double bonds are sp2 hybridized, so bond angle should have been 1200 but ring’s geometry is of regular hexagon and it means bond angle becomes 1200.
Strain= 1200−1200
Strain= 00
- 3-methylCyclopentene(IV): In 3-methylcyclopentene carbons forming double bonds are sp2 hybridized, so bond angle should have been 1200 but ring’s geometry is of regular pentagon and it means bond angle becomes 1080.
Strain= 1200−1080
Strain= 120
- Now if we compare the stabilities of cycloalkenes, the difference between stability of I and II is more than III and IV because strain difference in I and II is more than the III and IV
The correct answer is option “A” .
Additional Information : In spite of having 2π electrons, cyclopropene doesn’t show resonance because in it delocalization of electrons doesn’t take place.
Note: Hyperconjugation is the stabilizing interaction that results from the interaction of the electrons in a C-H σ-bond with an adjacent empty or partially filled p-orbital or a π-orbital to give an extended molecular orbital that increases the stability of the system.
