Question
Question: Which of the following statements is correct? A- Dipole moment 
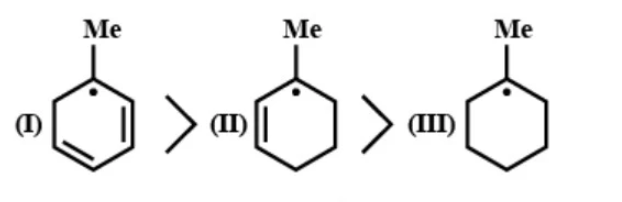
B- Stability of free radical
C- Basic strength: CH3O−>OH−>HS−
D- Basic and nucleophilic strength: I−>Br−>Cl−>F−
Solution
Verify the options and try to rule out incorrect options. Think about how the dipole moment is calculated. Comparing the magnitude of the dipole moment only does not consider the direction of the dipole moment. Think about the factors affecting the stability of free radicals. Think about the relation between base strength and their conjugate acid strength.
Complete step by step answer:
Let us verify the given options and find correct answers. In option A we are asked about dipole moments of di halo benzene compounds. Let dipole moments of C-Br and C-Cl bonds be a and b respectively. 1,4 dibromo benzene will have 0 dipole moment because dipole moments due to both C-Br bonds cancel out. In 1,2 dibromo benzene, the angle between 2 dipoles is 60 degrees. The resultant dipole moment will be the 1.7 times dipole moment of C-Br which is 1.7a in 1,3 dichlorobenzene angle between 2 dipoles is 120 degrees. The resultant dipole moment will have the same magnitude as of a single dipole moment.
Dipole moments of C-Cl and C-Br bonds all almost the same. 1.7(dipole of C-Br) will be greater than the dipole of C-Cl. Therefore, the given order is correct.
Free radicals will be more stable if there are more resonating structures. In the first compound there are 2 double bonds for resonance, in the second compound there is on double bond for resonance and in the third compound, there are no double bonds for resonance. So, the given order is correct.
In option C methyl alcohol is more acidic than water so conjugate base strength will be in reverse order. Methoxy ion will be less basic than hydroxide ion so the given option is incorrect.
In option D we acidic strength of hydrogen halides will increase down the group so the basic strength of conjugate bases will be in reverse order. Iodide ion will be less basic than bromide ion, bromide ion will be less basic than chloride ion and chloride ion will be less basic than fluoride ion. This option is also incorrect. So, the correct answer is “Option A and B”.
Note: As the angle between 2 dipoles increases the magnitude of net dipole moment will decrease. The nucleophilic strength of halide ions also depends on the nature of solvents. In polar protic solvents and polar aprotic solvents, the order of nucleophilicity will be different for halide ions.
