Question
Question: Which of the following statements are correct for the given reaction. 
A.Major product is a mixture of two enantiomers.
B.Less stable carbocation gives a major product.
C.Less stable free radicals give major products.
D.More stable free radicals give major products.
Solution
Here the given reactant is 2-methylbut-2-ene and this is reacted with peroxide and there is a formation of two products and that is 2-bromo-2-methylbutane and 2-bromo-3-methylbutane. And here, the peroxide acts as a catalyst. It is a chemical compound having a general structure, R−O−O−R. Here, R can be any element and the oxygen group acts as the peroxide group.
Complete answer:
Here, the given reactant is an alkene which is an unsaturated hydrocarbon. And this compound is showing antimarkovnikov’s rule. Because it contains one double bond. The hydrogen bromide acts as an electrophile hence it is added to the C-C double bond and there is a formation of two products which is 2-bromo-2-methylbutane and 2-bromo-3-methylbutane. Here, it takes place by the mechanism of free radical addition and it is relevant only for H-Br with hydrogen peroxide.
And here, the major product will be the mixture of two enantiomers and that is,
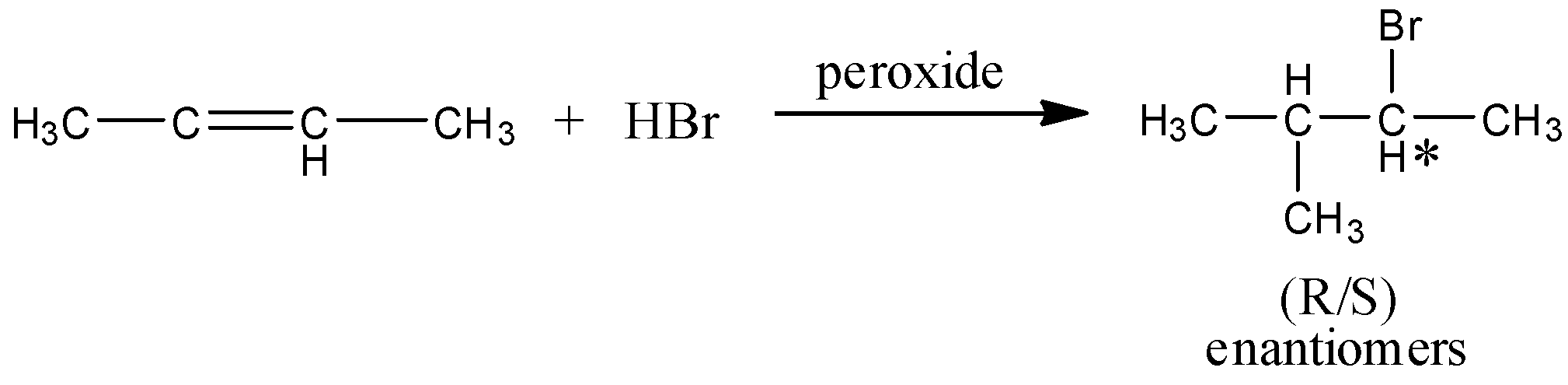
Hence, option (A) is correct.
According to anti markov rules, the less stable carbocation will not give the major product. Hence, the option (B) is incorrect.
Here, less stable free radicals will not give the major product. Hence, option (C) is incorrect.
In the case of antimarkov nikov’s rule, the more stable free radical gives the major product. Here, the reaction takes place in the term of free radical mechanism. And the reaction can be written as,
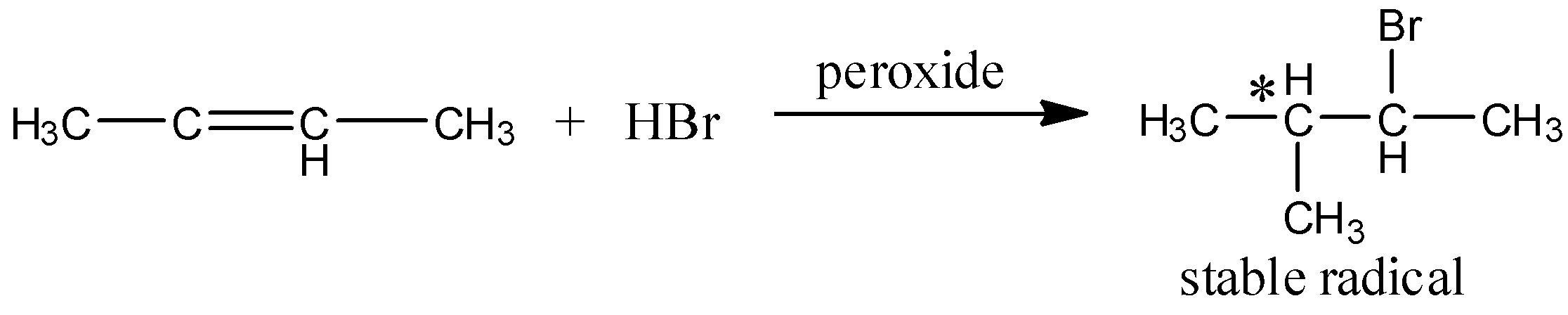
Hence, the option (D) is correct.
Note:
The given reaction can be explained by using the Markovnikov and anti markovnikov addition mechanism. Here, the hydrogen peroxide acts as a catalyst and it will help to break the H-Br bond and form the radicals. And here, the bromine is added to the primary carbanion. The bromine radicals added to the carbon of alkene compound which should be less substituted C atom and it is more stable.
