Question
Question: Which of the following statements about \(H_3PO_4\) is/are correct? This question has multiple cor...
Which of the following statements about H3PO4 is/are correct?
This question has multiple correct options
A.It is a strong tribasic acid
B.It is prepared by acidifying an aqueous solution of borax
C.It has a layer structure in which BO3 units are joined by hydrogen bond
D.It does not act as proton donor as it act as Lewis acid an accept the hydroxyl group
Solution
The boric acid is a weak acid which acts as an acid by forming H3O+ ion.Boric Acid is a monobasic Lewis acid with the chemical formula H3BO3. It is an acid-containing four atoms of oxygen, one atom of phosphorus, and three atoms of hydrogen. Boric acid is also known as acidum boricum, hydrogen borate, boracic acid, and orthoboric acid.
Complete step by step answer:
Let’s start by understanding the structure of H3BO3, The structure is given below:
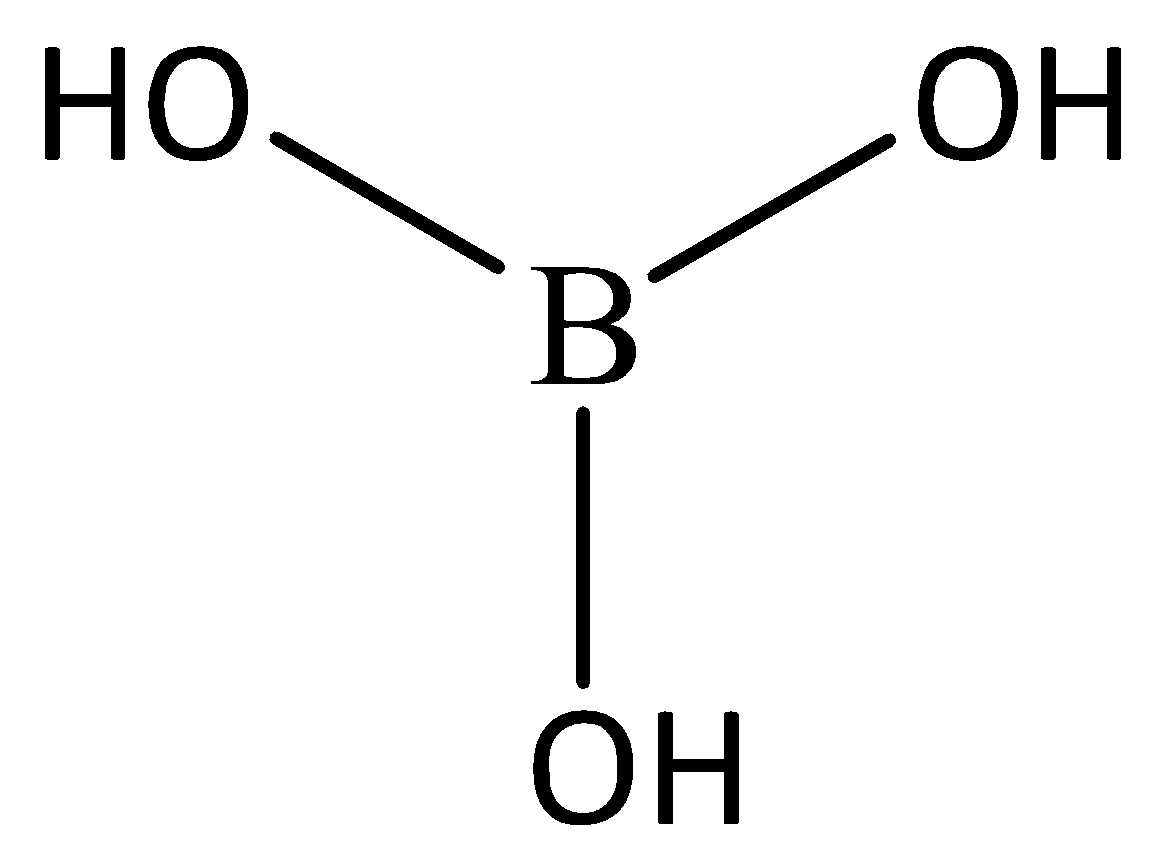
Looking at the structure we can determine that it is a planar structure, In this the crystalline structure is having the layers of BO3 units which are joined together by hydrogen bonding. Hence, option C. is correct.
Now moving to check the option A, the H3BO3 is a weak monobasic acid, so clearly the option A is incorrect.
As for the preparation of H3BO3, in the preparation process of H3BO3 the borax is reacted with a mineral acid in aqueous medium. Borax is sodium tetraborate decahydrate and the reaction for the preparation of boric acid is as follows:
Na2B4O710H2O + 2 HCl →4 B(OH)3[or H3BO3] + 2 NaCl + 5 H2O
Hence, the option C is also correct.
Coming to option D, The boric acid acts as a lewis acid by accepting the hydroxyl ion and forming H3O+ ions in the solution which results in an acidic medium.
B(OH)3 + 2H2O→[B(OH)4] - + H3O +
Hence, option D is also correct.
So, the correct answer to this question is options B, C, D.
Note:
Boric acid has a wide range of applications. The applications range from its use as an antiseptic, insecticide, flame retardant, neutron absorbent or precursor for other reactions. It exists in the form of a colourless crystal or in the form of white powder which is dissolved in water to get the acid.
