Question
Question: Which of the following statement(s) is/are wrong? A)The malting point of trans-but-2-ene is great...
Which of the following statement(s) is/are wrong?
A)The malting point of trans-but-2-ene is greater than that of the cis-isomer due to symmetrical packing in the crystal lattice of the trans-form.
B)The boiling point of cis-but-2-ene is greater than that of the trans-isomer due to steric strain in the cis-isomer because of van der Waals repulsion force of large group on the same side of the double bond and the cis-isomer becomes less stable because of the increasing repulsive force.
C)The boiling point of the cis-isomer is greater than that of the trans-isomer due to the relatively high polarity of the cis-isomer compared to its trans-isomer.
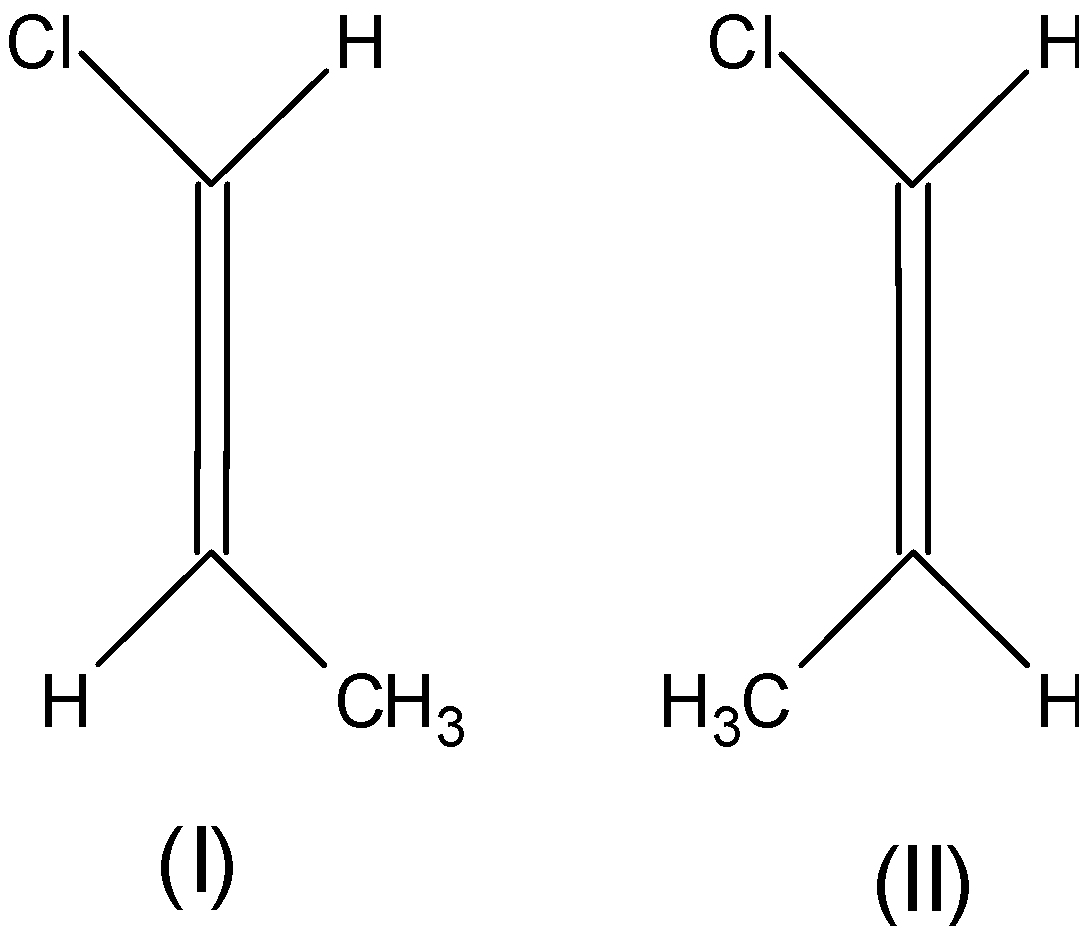
D) Refer Image
The boiling point of (I) is greater than (II)
Solution
Geometrical isomers are the type of isomers where the compounds with the same molecular formula differ in the arrangement of groups concerning double bond. This is Cis and trans-isomers.In cis isomer the bulky group are on the same side causes the higher repulsion and thus easily separate out.The trans isomers are tighly packed.
Complete step by step answer:
Isomers are defined as the compounds with the same molecular formula but have a different arrangement of the atoms. These compounds differ in physical properties.
Geometrical isomers are the type of isomers where the compounds with the same molecular formula differ in the arrangement of groups concerning double bonds. This is Cis and trans-isomers.
The cis and trans isomers have different physical properties due to the difference in the arrangement of atoms in space or because of the dipole moment of the molecule.
Let's consider the boiling and melting point of the molecules for cis and trans.
Let's take an example of but-2-ene. The but 2-ene exist in two isomeric forms.One as a cis but-2-ene and the other as the trans-but-2-ene.
It has been found that the boiling point for the cis isomer boils at the temperature 40and trans boils at the temperature of 10 Celcius. If we look at the structures of cis isomer is more polar due to the configuration of methyl groups on the same side of the double bond. The two methyl groups donate its electron density to the relatively negative double bond. Thus the methyl group acquires a slightly positive charge. The resultant moment of the dipole is non zero since the electron density moves in one direction.
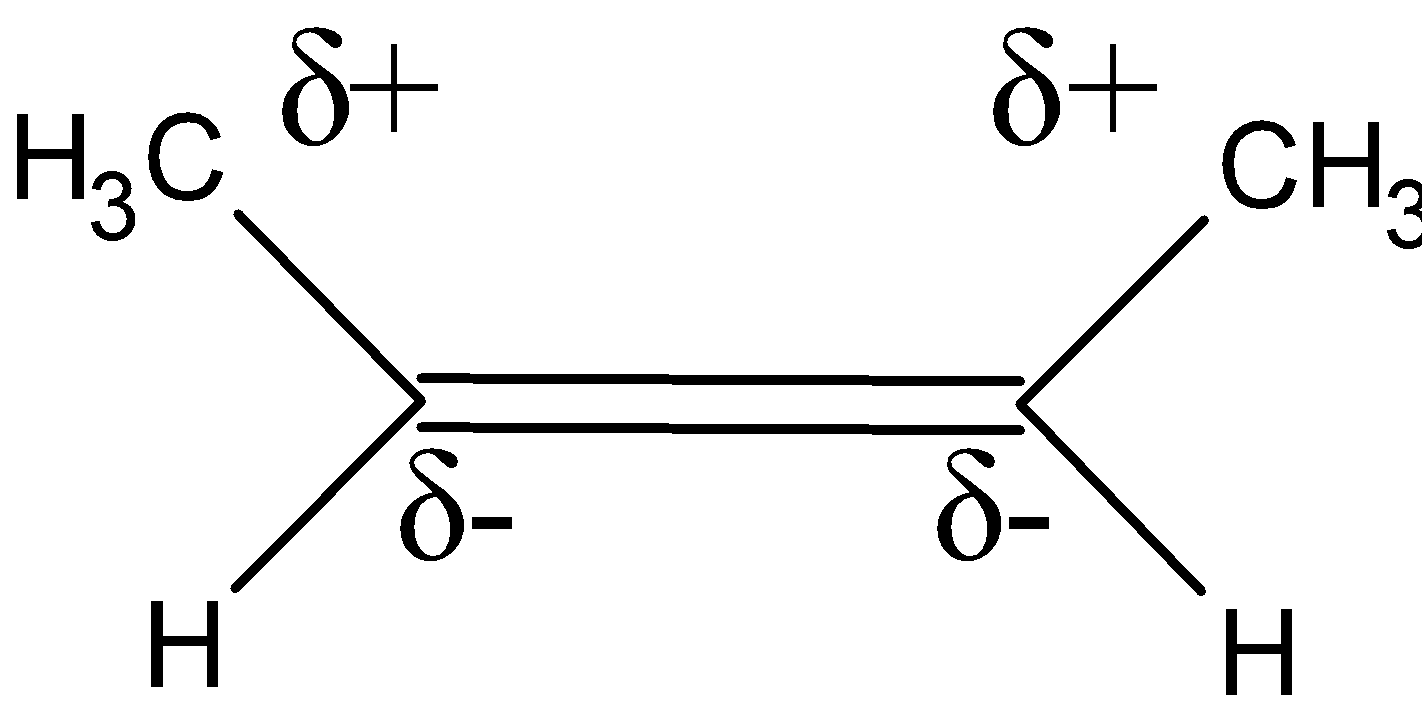
However, for the trans isomer, the electron-donating methyl groups are opposite to each other.they release their electron density towards the carbons of the double bond. Since, both the methyls are opposite to each other they cancel out their dipole moment, and thus the resultant dipole moment for the trans isomer is zero. It is an asymmetrical molecule and-polar in nature.
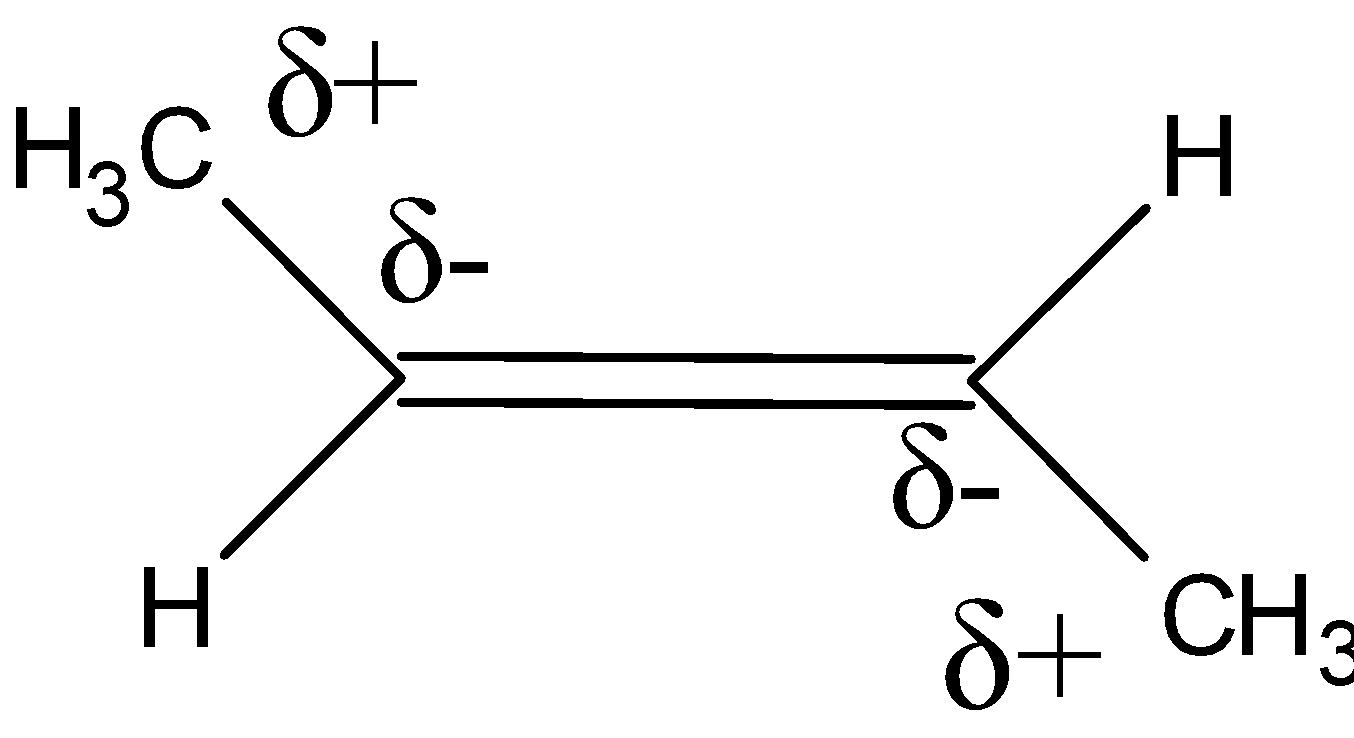
The slight positive charge at the top of the molecule is balanced by the equivalent positive charge at the bottom of the molecules. Similarly negative charge on the left of the molecule is balanced by a negative charge on the right.
This means that the only possible interactions which exist in trans isomers are the van der Waals dispersion forces. since the molecules require less energy to overcome the forces and separate the molecules.
But in cis isomer is polar and hence the forces of attraction between the molecules are higher .thus require large energy as compared to trans to separate the molecules. Thus the boiling point is greater.
The melting point follows a different trend than the boiling point. The general trend is trans have a high melting point than the cis. The reason is the trans isomer can pack tightly in the solid form. This is because the dispersion force or the dipole-dipole interactions are relatively weaker than the intermolecular forces can act on short distances in trans isomers. Molecules pack itself close to each other thus required high energy to separate hence melting point for trans is more than cis isomer.
In the case of cis-isomer, both bulky groups are at the same as the double bond results in the repulsion between groups. The van der Waals forces of groups act on the same side of the double bond. Thus the cis isomer cannot hold each other tightly. Because of this, they have less melting points than trans. The repulsion between the crowding group destabilizes the isomer thus it increases the boiling point of the cis isomer.
Thus option (A),(B), and (C) are correct statements and (D) is incorrect.
Hence, the correct option is (D)
Note: Cis, as the name suggests, has a bulky group on the same side. And in trans, the groups lie opposite to each other. In trans-isomer, the molecules perfectly fits with each other hence densely packed while for cis the spacing causes the higher boiling point.
