Question
Question: Which of the following statement(s) is/are correct? A. \({{\text{O}}_{3}}\) molecule is bent B. ...
Which of the following statement(s) is/are correct?
A. O3 molecule is bent
B. Ozone is violet-black solid
C. Ozone is diamagnetic gas
D. ONCl and ONO are not isoelectronic
Solution
For this problem, we have to draw the structure of ozone and write its electronic configuration to determine whether it is paramagnetic or diamagnetic. Also, we have to count the electrons of ONCl and ONO to determine whether they have the same number of electrons or not.
Complete step by step answer:
- In the given question, we have to determine whether the given statements are true or not.
- So, firstly we have to draw the structure of ozone so that we can determine whether the geometry of the ozone molecule is bent or not.
- The structure of the ozone is:
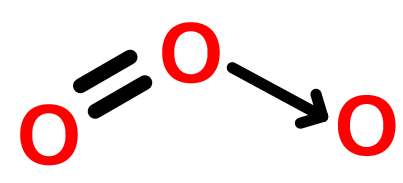
- So, the structure of the ozone is considered as bent.
- Now, we will write the electronic configuration of the ozone with the help of the Molecular orbital theory i.e. KK (σ 2s)2 (σ∗ 2s)2 (σ 2pz)2 (π 2px2 = π 2py2) (π∗ 2px2 = π∗ 2py2) (σ* 2pz)2 (σ 3s)2 (σ* 3s)2
- Also, we know that ozone is a gas which has three oxygen molecules and has a pungent smell along with it having the colour of violet-black.
- Now, to determine that the molecules ONCl and ONO are isoelectronic or not we have to count the number of electrons.
- because isoelectronic species are those species which have an equal number of the electrons and if they have a different number of electrons they will not be considered as the isoelectronic species.
- So, in ONCl, there is one oxygen which has 8 electrons, one nitrogen which has 7 electrons and one chlorine which has 17 electrons.
- The total number of the electron will be 32.
- Now, in ONO there are two oxygen atoms which will have 16 electrons and one nitrogen atom which will have 7 electrons.
- So, the total number of electrons will be 24.
- Hence, ONO and ONCl are not isoelectronic as proved.
So, the correct answer is “All correct”.
Note: Ozone is a major compound which is widely used as an ozone shield to protect the earth from the ultraviolet rays of the sunlight. UV is harmful to humans as it can cause skin irritation or skin cancer.
