Question
Question: Which of the following statement(s) is/are correct? (A) Dipole moment of diborane is zero (B) Di...
Which of the following statement(s) is/are correct?
(A) Dipole moment of diborane is zero
(B) Diborane is lewis acid
(C) Diborane has incomplete octet
(D) Diborane has four 2C−2e− bond
Solution
Lewis acid is a compound that can accept a pair of electrons from Lewis base. 2C−2e− stands for two centered two electron bonds. Diborane contains two banana bonds. Molecular formula of diborane is B2H6.
Complete answer:
Let’s check all the statements one by one.
A)
- To check the dipole moment of the molecule, we first need to identify the shape and structure of the molecule. The structure of diborane is shown as below.
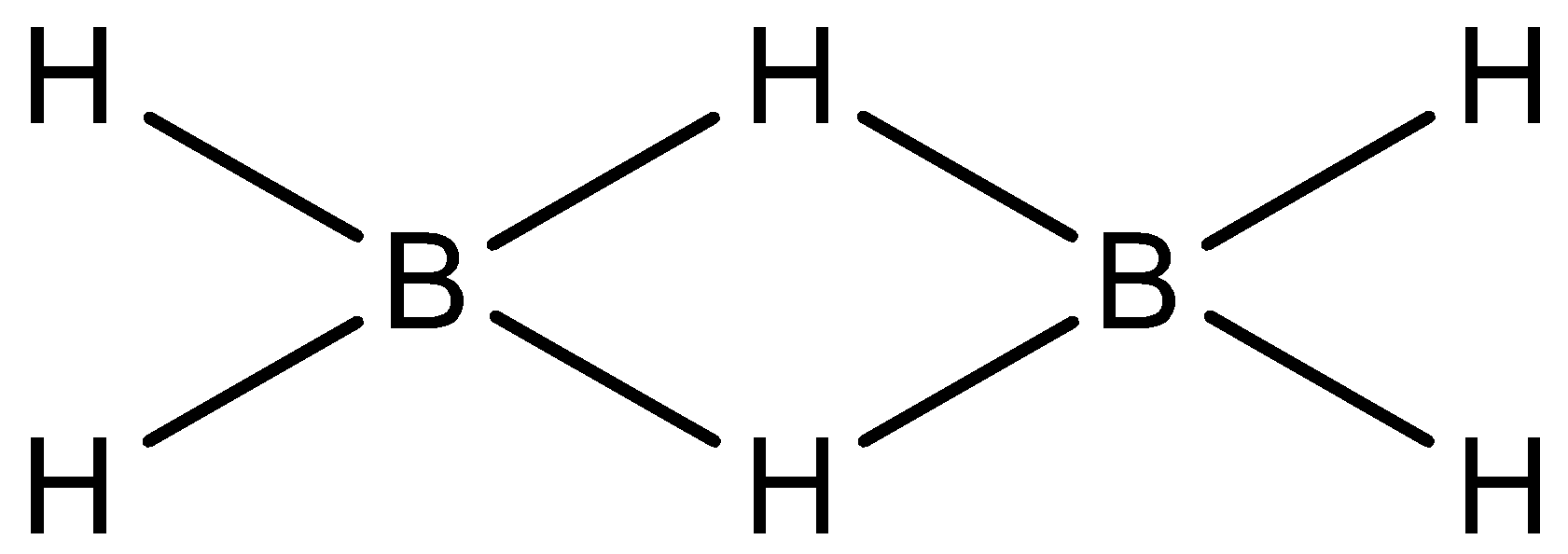
Now, we need to find whether the net dipole moment is present in the molecule or not. We know that B has slight positive charge and H has a slight negative charge in the B-H bond. So, this polarity will generate a small dipole moment for that particular bond but as the molecule is symmetrical, the small dipoles generated will be cancelled out by opposite dipoles. So, the net dipole moment of this molecule will be zero. So, statement (A) is correct.
B)
We know that the molecular formula of diborane is B2H6. So, it contains two B atoms. Let’s see electronic configuration of B to check whether it will act as a lewis acid or not.
- Electronic configuration of B is 1s22s22p1 and hydrogen has one electron. So, we can say that this compound will have a total of 2(5) + 6 = 16 electrons. So, we can say that this will accept a pair of electrons and will obtain a stable 18 electron structure. Lewis acid is a compound that can accept a pair of electrons from lewis base. Thus, this statement is correct.
C)
Let’s see electronic configuration of B in order to check whether it has a complete octet or not.
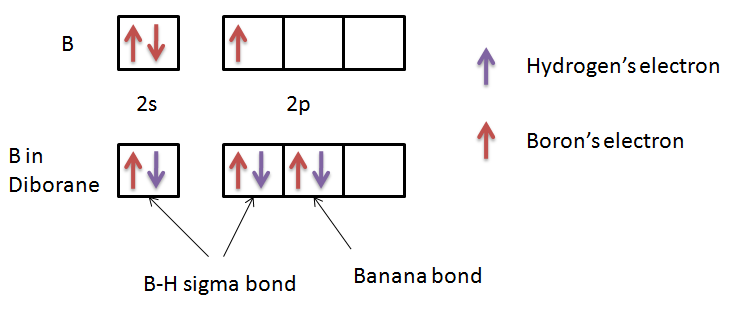
We can see in the figure that there are only 6 electrons present in the valence orbital of the boron. Thus, we can conclude that the octet of boron is not complete in diborane. So, this statement is also true.
D)
2C−2e− bonds stand for two centered two electron bonds. We know that generally all the bonds are two center-two electron bonds. The bonds in which hydrogen atoms are bonded with only one boron atom are called 2C−2e− bonds. Here, we can see four of those bonds. So, we can say that this statement is also true.
Therefore all options (A), (B), (C) and (D) are correct.
Note:
Remember that net dipole moment of a compound is the sum of all dipoles present in the given compound. Symmetrical molecules do not have net dipole moments. Do not get confused between Lewis acid and Lewis base in which electron acceptor is lewis acid and electron donor is lewis base.
