Question
Question: Which of the following statement is correct?...
Which of the following statement is correct?
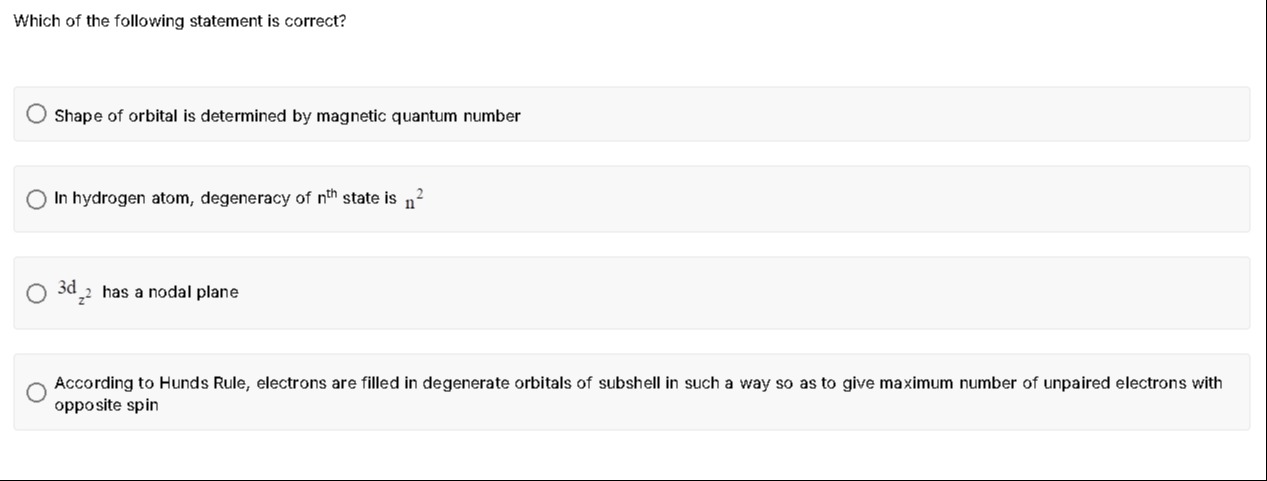
Shape of orbital is determined by magnetic quantum number
In hydrogen atom, degeneracy of nth state is n2
3dz2 has a nodal plane
According to Hunds Rule, electrons are filled in degenerate orbitals of subshell in such a way so as to give maximum number of unpaired electrons with opposite spin
In hydrogen atom, degeneracy of nth state is n2
Solution
The shape of an atomic orbital is determined by the azimuthal quantum number (l). The magnetic quantum number (ml) determines the orientation of the orbital in space. In a hydrogen atom, the degeneracy of the nth state is n2 because all orbitals with the same principal quantum number (n) have the same energy. The 3dz2 orbital has two conical surfaces of nodes, not a nodal plane. Hund's rule states that electrons fill degenerate orbitals with parallel spins to maximize multiplicity, not opposite spins.
