Question
Question: Which of the following species is not electrophilic in nature? A) \(C{{l}^{+}}\) B) \(B{{H}_{3}...
Which of the following species is not electrophilic in nature?
A) Cl+
B) BH3
C) H3O+
D) NO2+
Solution
Electropositive elements are those which are electron deficient species and electronegative elements are those which are rich in electrons and the answer can be approached based on this fact.
Complete answer:
In the chapters of chemistry, we have studied the basic concepts of chemistry which tells about the properties of the elements that include electropositivity (electrophiles) and also the electronegativity (nucleophiles) and the neutral atoms and their definitions.
Let us now see in detail about these and deduce the required answer.
- Electrophiles are those which are electron deficient and do not have any electrons for the donation.
- Now among the given options, let us see each option in detail.
A) Cl+ is an electrophile as it does not have any electrons or lone pairs in it. It does not independently exist but in the presence of Lewis acids like aluminium chloride, they are generated as chloronium ions.
B) BH3 is an electrophile because it has the vacant p-orbitals and there also exist a strong partial positive charge on the boron due to the extremely electronegative fluorine atoms which are bound covalently. The structure is shown below,
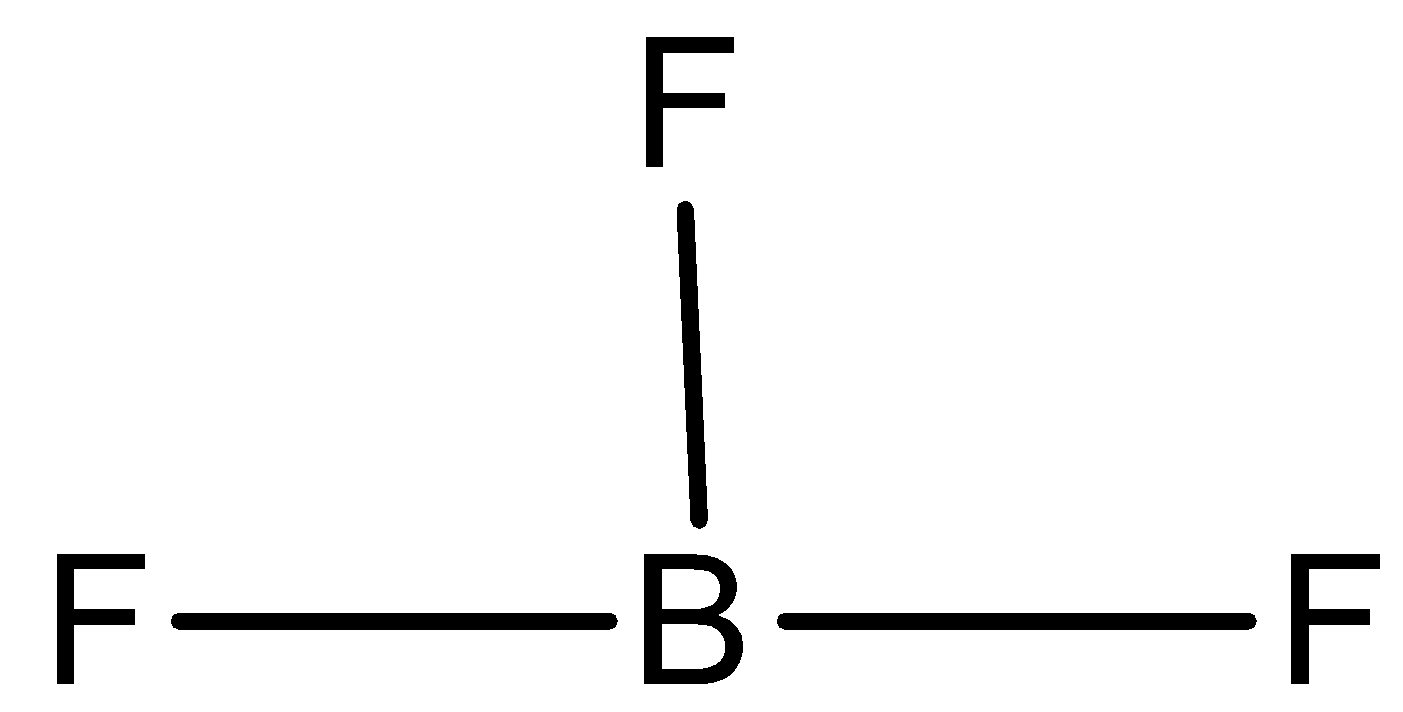
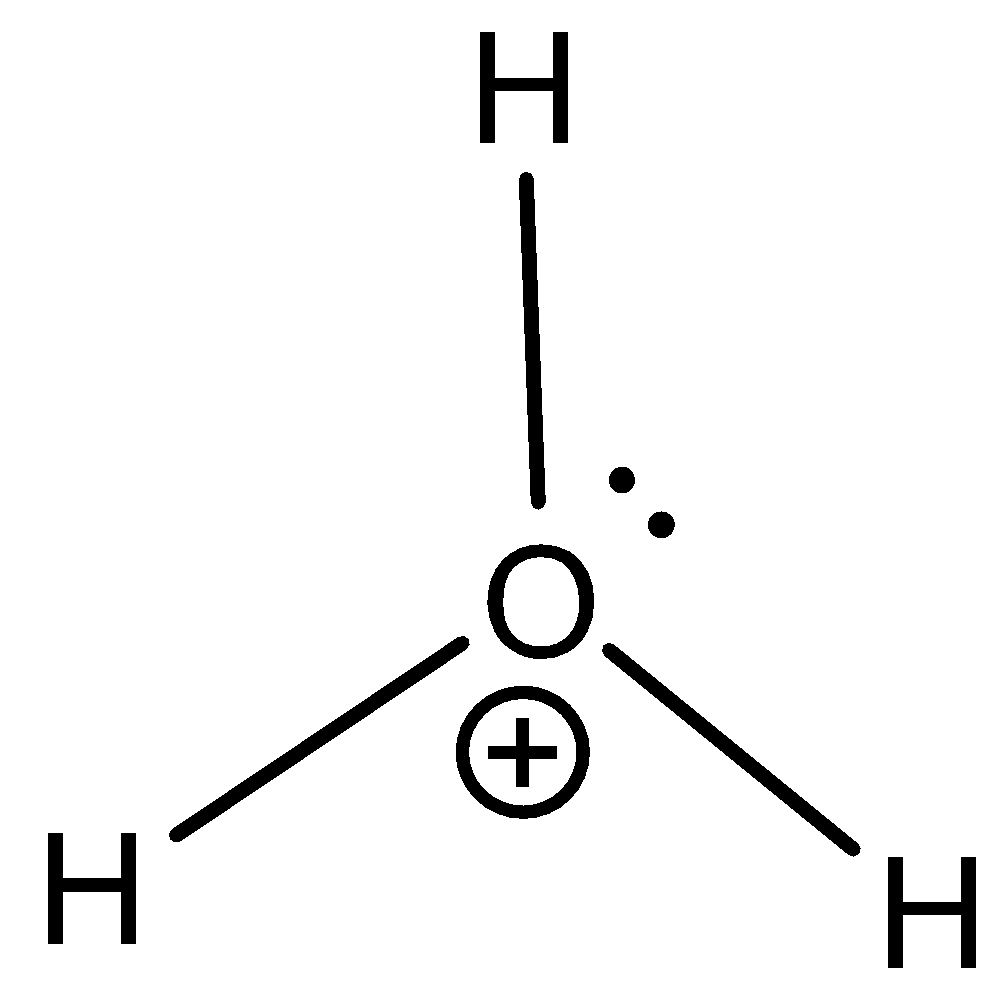
C) H3O+ has a lone pair of electrons present in it for the donation and thus is not electron deficient in nature and therefore is not an electrophile. The structure is shown below,
D) NO2+ does not have any octet configuration or octet around it and thus it is an electrophile and also the lone pair of electrons on nitrogen is used up for the donation. The structure is shown below,
Hence, the correct answer is option C)
Note:
Note that if the molecule given is neutral or is having the overall charge of positive, this does not mean that it is an electrophile because even the lone pair of electrons is to be taken into consideration.
