Question
Question: Which of the following species is/are polar in nature? A) \(Xe{{F}_{4}}\) B) \(Xe{{F}_{6}}\) ...
Which of the following species is/are polar in nature?
A) XeF4
B) XeF6
C) XeOF4
D) XeF5−
Solution
The answer to this question includes the fact about the polarity measurement which is based on the dipole moment where if the value of this dipole moment is not zero then that compound is polar in nature.
Complete answer:
In the lower classes of chemistry, we have studied the basic concepts of chemistry that deal with the inorganic part that includes the definition for polarity and which is further based on the dipole moment of the compound given.
Now, let us see this in detail so that we will know which among the following will be polar in nature.
Now, the dipole moment denoted by μ is nothing but defined as the quantity which describes two opposite charges that are separated by a certain distance ‘r’.
- It is the measure of net molecular polarity which is the magnitude of the charge at either end of the molecular dipole times the distance between them.
- Polarity therefore can be checked by drawing the structures of given compounds and if the dipole moment μ is zero then the compound is polar.
In option A) XeF4, the structure is
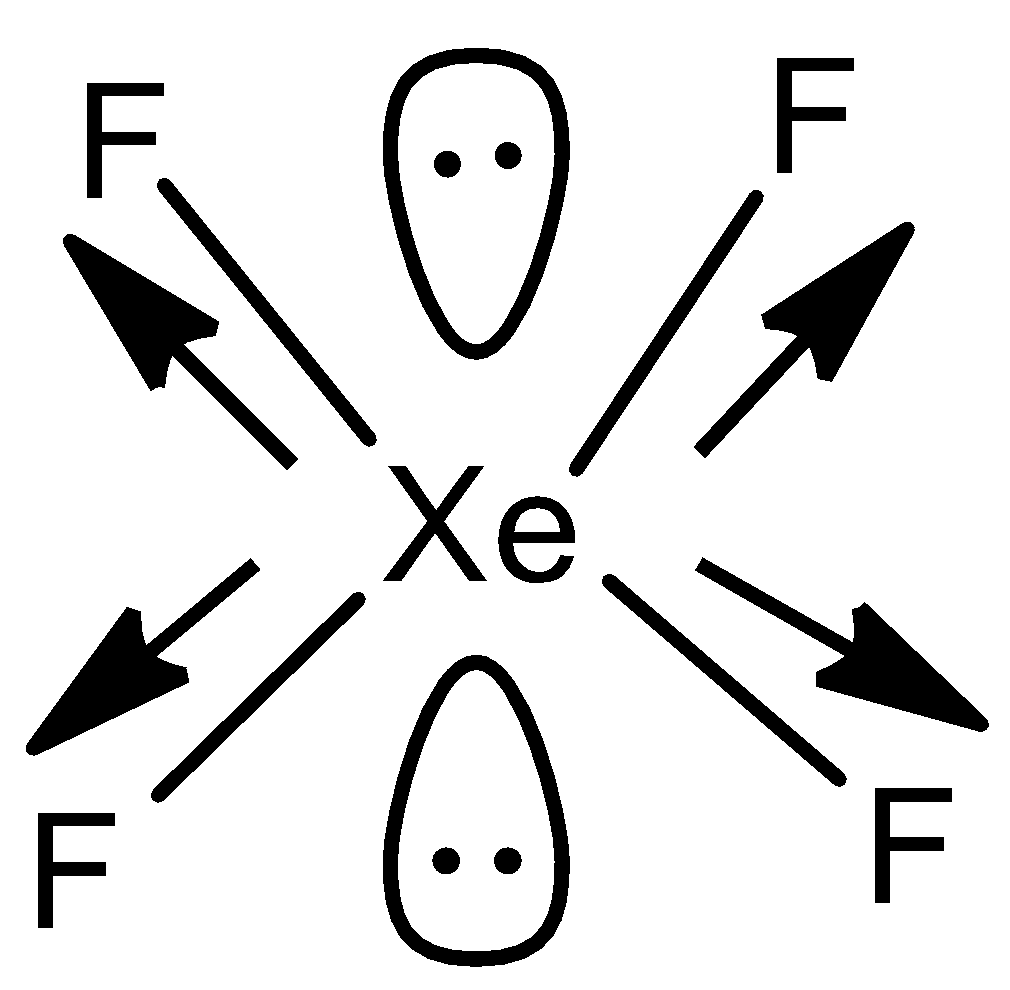
Here, the compound has square plane structure with hybridisation of sp3d2 and here the dipole moments cancel each other as shown with arrows and thus the value will be μ=0
In option B) XeF6, the structure will be,
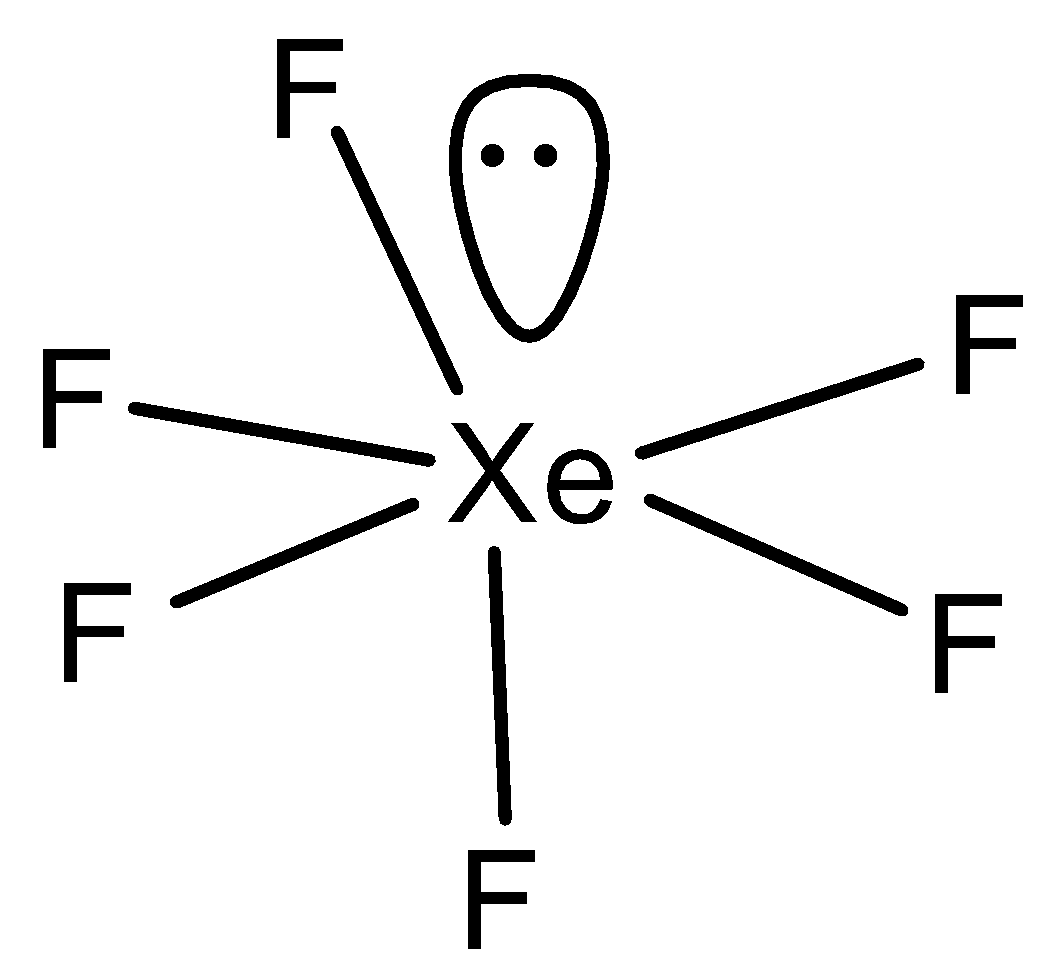
Here, this compound has distorted octahedral structure and since it is distorted, the dipole moment cannot become zero and thus μ=0
In option C) XeOF4, the structure will be,
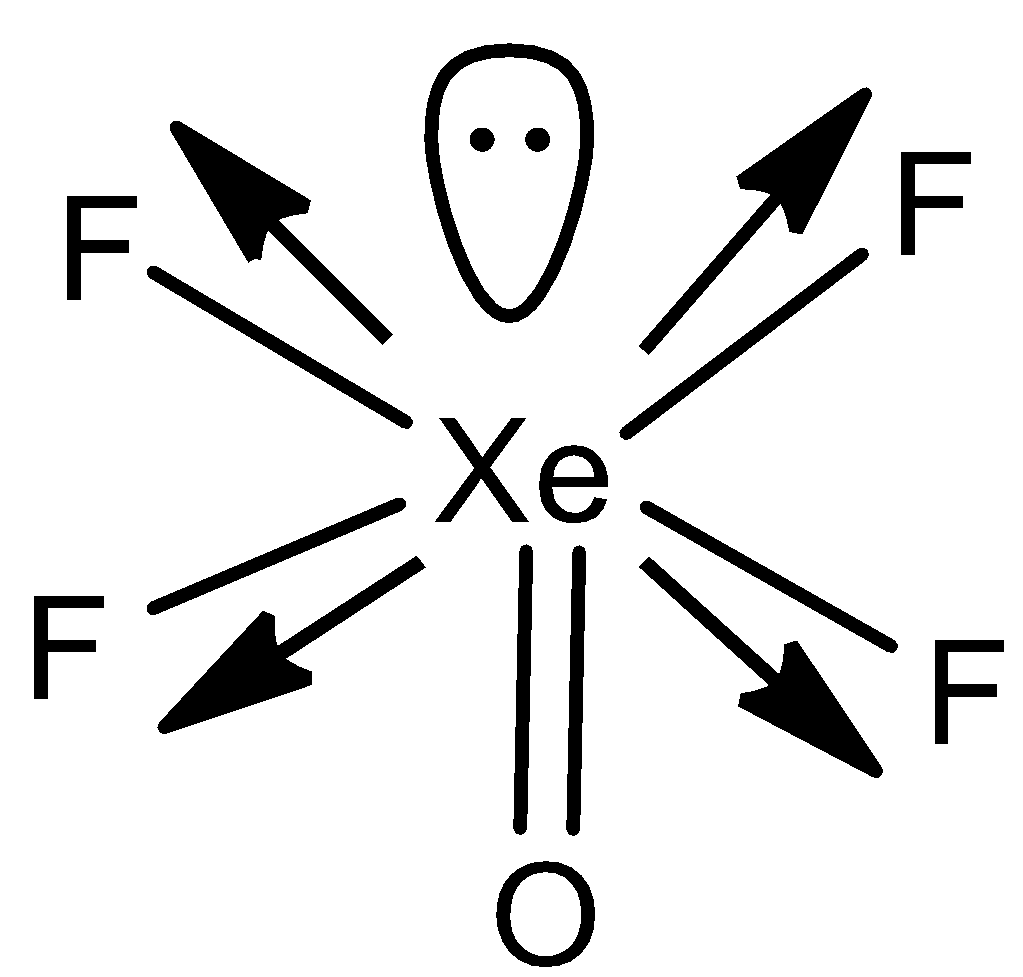
In this case, the dipole moments of fluorine will cancel each other but that of oxygen and lone pair of electrons cannot cancel and therefore μ=0 in this case.
In option D) XeF5−, the structure is,
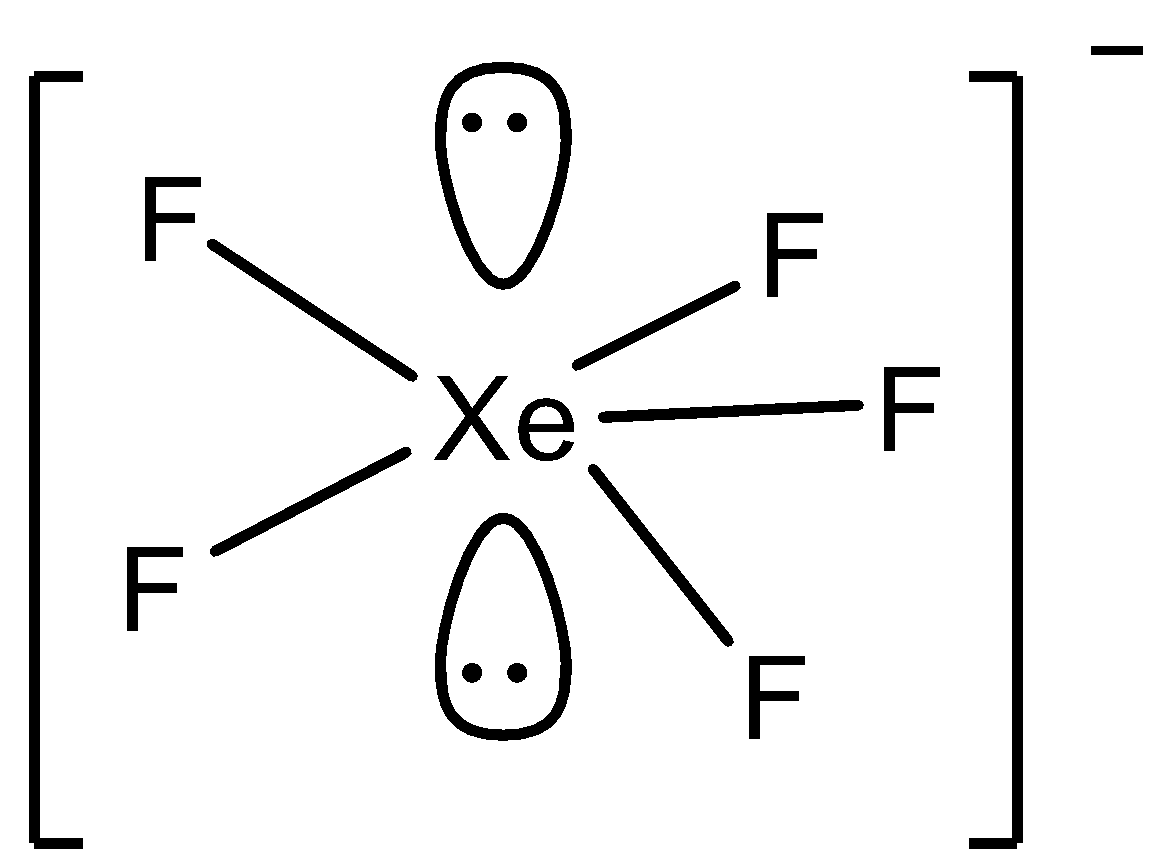
Even in this structure there is no complete cancellation of the dipole moment since the geometry is pentagonal bipyramidal. Thus μ=0 in this case too.
Therefore, the correct answer is option B), C) and D).
Note:
Note that to know the polarity, structures of the given compound plays an important role and be thorough with the structures of common compounds and also sometimes the multiple choice question can have several correct answers and do not stop the calculation when one answer obtained is correct.
