Question
Question: Which of the following species have undistorted octahedral structures? 1\. \[S{F_6}\] 2\. \[PF_6...
Which of the following species have undistorted octahedral structures?
1. SF6
2. PF6−
3. SiF62−
4. XeF6
A. 2,3 and 4
B. 1,3 and 4
C. 2 and 3
D. 1, 2 and 3
Solution
In the undistorted octahedral shape of the molecule the central atom does not possess any lone pairs as the valence electron is used in forming six chemical bonds with six fluorine atoms.
Complete step by step answer:
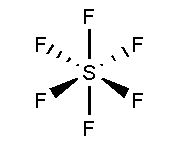
In SF6, the Sulphur atom is surrounded by six fluorine atoms. The atomic number of Sulphur is 16 and the electronic configuration is [Ne]3s23p4. The atomic number of fluorine is 9 and the electronic configuration of [He]2s22p5. Total 12 valence electrons form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It is arranged in undistorted octahedral shape.
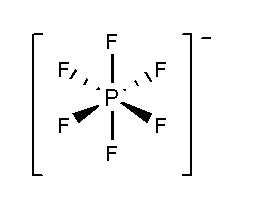
In PF6−, the phosphorus atom is surrounded by six fluorine atoms. The atomic number of phosphorus is 15 and the electronic configuration is [Ne]3s23p3. The atomic number of fluorine is 9 and the electronic configuration of [He]2s22p5. Total 11 valence electrons and one negative charge form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. According to VSEPR theory PF6−show undistorted octahedral structures
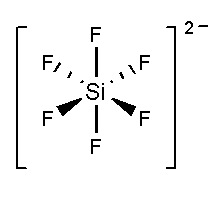
In SiF62−, the silicon atom is surrounded by six fluorine atoms. The atomic number of silicon is 14 and the electronic configuration is [Ne]3s23p2. The atomic number of fluorine is 9 and the electronic configuration of [He]2s22p5. Total 10 valence electrons and two negative charges form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It forms an undistorted octahedral shape.
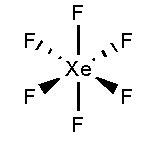
In XeF6, the xenon atom is surrounded by six fluorine atoms. The atomic number of xenon is 54 and the electronic configuration is [Kr]4d105s25p6. The atomic number of fluorine is 9 and the electronic configuration of [He]2s22p5. Total 12 valence electrons form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It forms an octahedral shape.
Therefore, the correct option is D
Note:
In XeF6 as the valence electrons in xenon is 8 out of which six forms bond and the remaining two electrons and present as lone pairs and located in the sp3d3hybrid orbital. Therefore it forms a distorted octahedral complex.
