Question
Question: Which of the following shows \[ds{{p}^{2}}\] hybridisation and a square planar geometry? A. \(S{{F...
Which of the following shows dsp2 hybridisation and a square planar geometry?
A. SF6
B. BrF5
C. PCl5
D. [Ni(CN)4]2−
Solution
We have to find out the hybridisation, which means when different atomic orbitals are combined having different energies to give the equivalent orbitals. Here we will see the combination of one d orbital, one s orbital and two p orbitals to give the dsp2 hybridisation and square planar geometry.
Step by step solution:
- We will find the electronic configuration of Ni in the compound [Ni(CN)4]2−,
- The electronic configuration of Ni is-1s22s22p63s23p64s23d8
- And the electronic configuration of Ni2+will be-1s22s22p63s23p64s03d8
- We can represent the valence bond representation as:
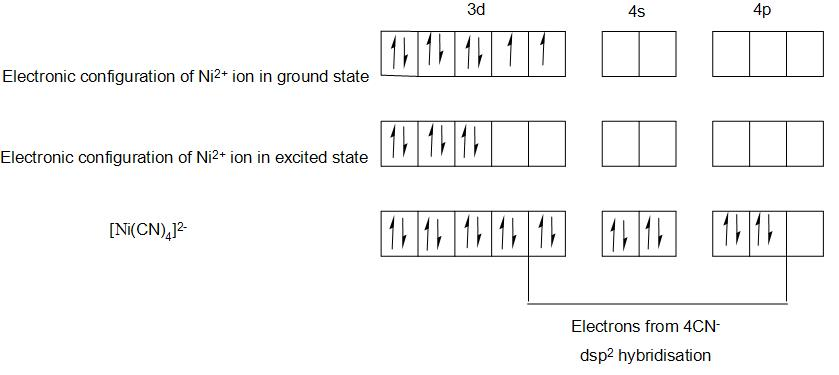
- Here we have filled the 8 electrons of Ni2+ in 3d orbitals, the two unpaired electrons in the ground state of ion, will pair up in the excited state , due to the pairing energy supplied by the formation of strong bonds in the complex.
- This makes one of the 3d orbitals empty. By this we can see above that there are no unpaired electrons and hence the compound will be diamagnetic.
- Here we can see that 4 CN− ions will form a strong bond in the complex.
- And here the central metal ion undergoes dsp2 hybridisation and the complex ion takes square planar geometry.
- Therefore, we can conclude that the correct option is(d) that is [Ni(CN)4]2− shows dsp2 hybridisation and a square planar geometry.
- As we have seen from the above valence bond representation that there are no unpaired electrons present and hence it is diamagnetic, we can also say that it has zero magnetic moment.
Note:
- In presence of any strong field ligand like CN− , all the electrons are paired up, and in the presence of weak field ligands electrons are not paired up.
- We can calculate the magnetic moment, hybridisation, geometry, and magnetic nature from the valence bond representation.
