Question
Question: Which of the following show geometrical isomerism?  A) a,b,d.
A) a,b,d.
B) a,c,d.
C) a, b, c.
D) c, b, d.
Solution
we discuss about geometrical isomers as,
On the off chance that there is a confined turn in a particle there emerges geometrical isomerism. Geometrical isomers are otherwise called Cis-trans isomerism.
If the two atoms secured same side of the atom then it is called as cis isomers.
If the two particles secure the inverse side of the atom then it is called trans isomers.
Complete step by step answer:
As we know that the geometrical isomerism is appeared along limited bond when the four gatherings connected make cis and trans.
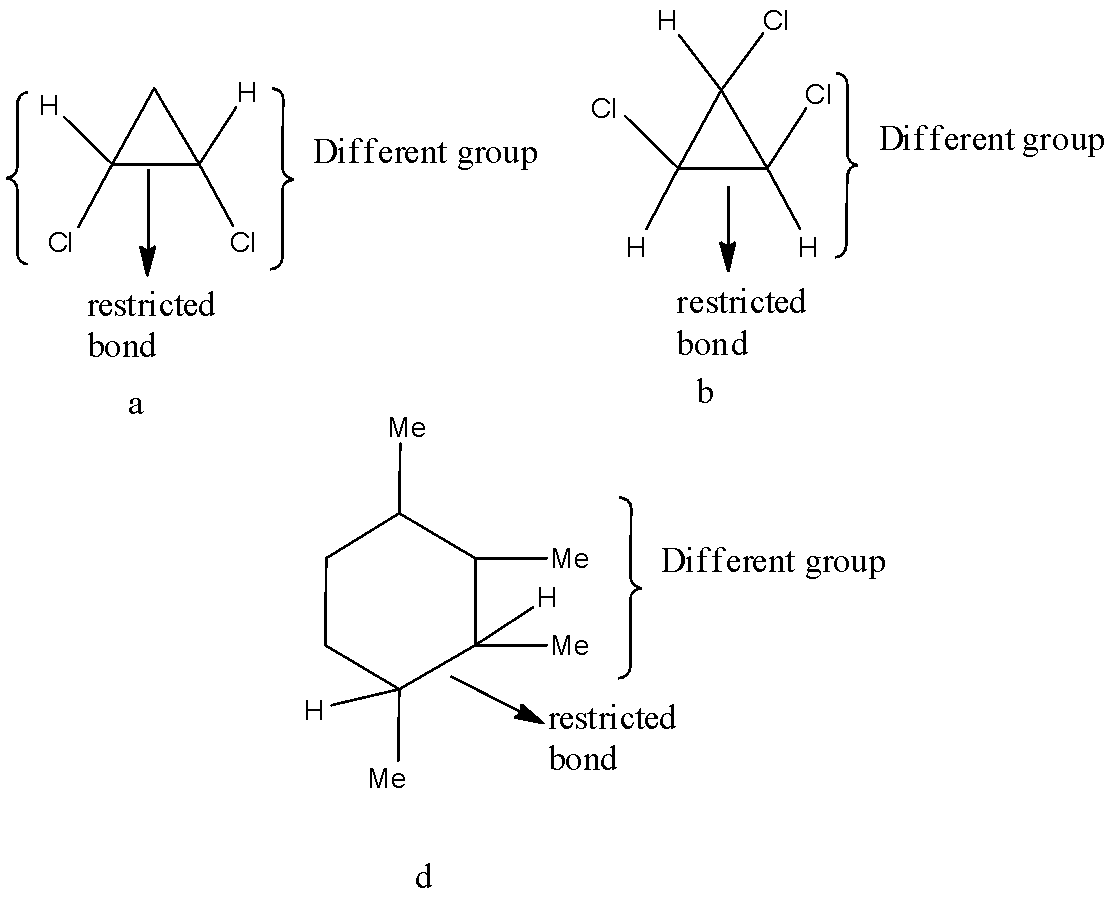
Thus, the correct answer to the question is option A.
Additional note:
We must need to know that the cis–trans isomerism, otherwise called geometrical isomerism or configurational isomerism, is a term utilized in natural science. With regards to science, cis shows that the useful gatherings are on a similar side of the carbon chain while Tran’s passes on that utilitarian gatherings are on rival sides of the carbon chain. Cis-trans isomers are stereoisomers, that is, sets of atoms which have similar equation yet whose utilitarian gatherings are turned into an alternate direction in three-dimensional space. It isn't to be mistaken for E–Z isomerism, which is an outright stereo chemical depiction.
Note: We must have to know that the cis and Trans isomers happen both in natural particles and in inorganic coordination edifices. Cis and trans descriptors are not utilized for instances of conformational isomerism where the two geometrical structures effectively interconvert, for example, most open-chain single-fortified structures; all things considered, the expressions "syn" and "against" are utilized. The expression "geometrical isomerism" is considered by IUPAC to be an old equivalent word of "cis–trans isomerism".
