Question
Question: Which of the following show diastereomers? (This question has multiple correct options) A. !...
Which of the following show diastereomers?
(This question has multiple correct options)
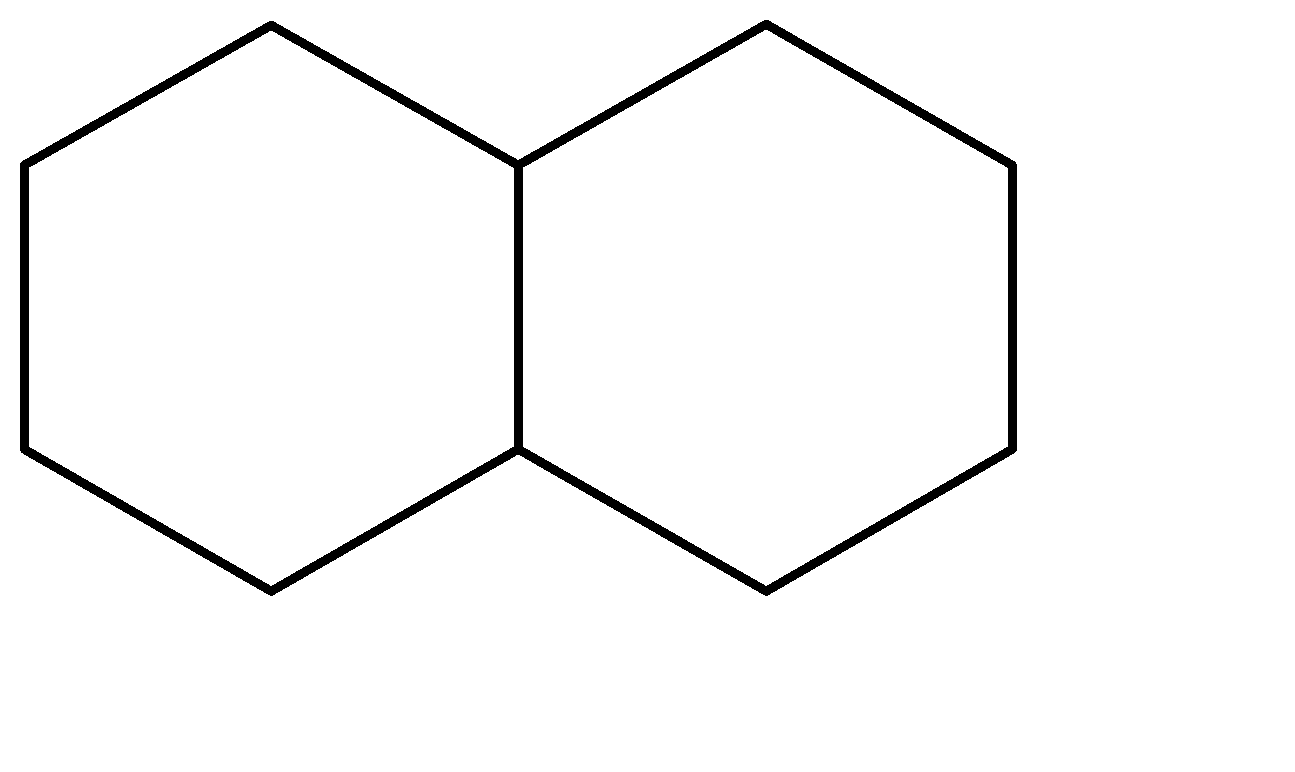
A.
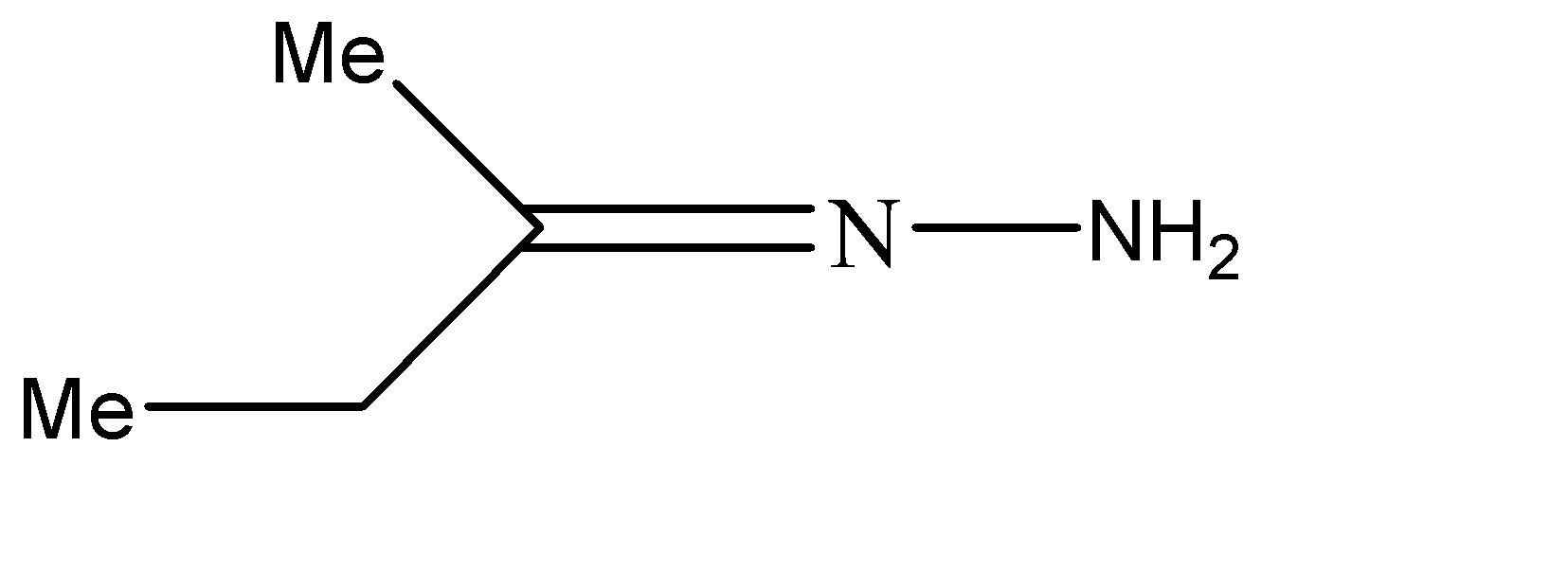
B.
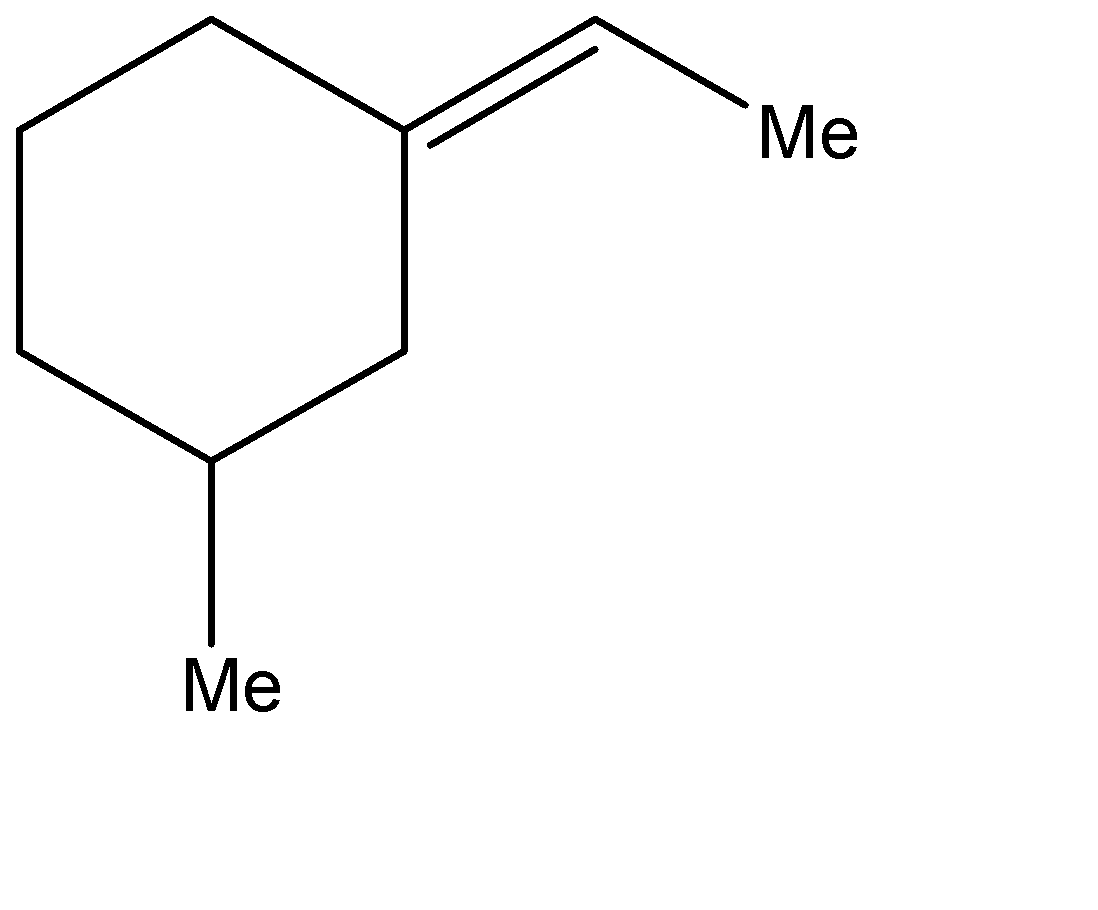
C.
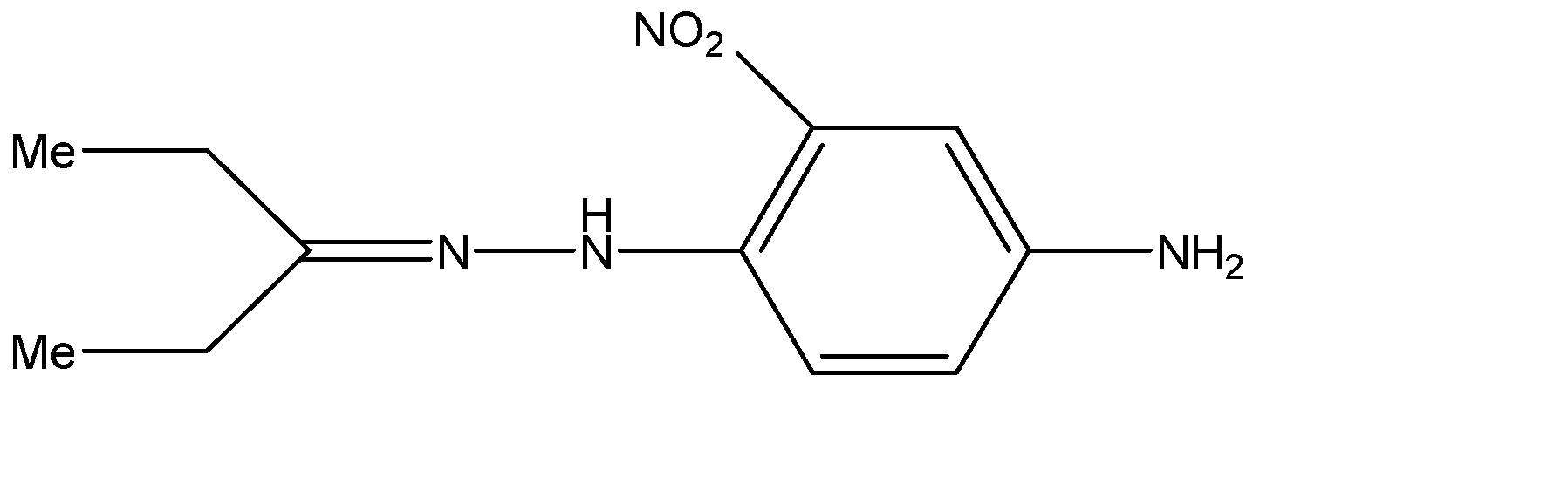
D.
Solution
Isomerism, stereoisomers and diastereomers are all interrelated and interconnected concepts. In order to understand diastereomers, we must first understand isomerism and stereoisomerism.
Complete step by step solution:
-In order to understand what a diastereomer is, let us discuss a few basic terms.
Isomers can be defined as different compounds having the same molecular formula.
-Stereoisomers are an iteration of isomers. In stereoisomers, the composition and arrangement remain the same, while the orientation of the parts of the molecule differ in 3-dimensional space. There are 2 types of stereoisomers: enantiomers and diastereomers.
-Diastereomers are stereoisomers that are not mirror images of one another and cannot be superimposed or visually identically placed over one another. Stereoisomers which have two or more stereo centres can be called as diastereomers.
-On the basis of these definitions, we can understand whether or not the given compounds can exhibit diastereomers.
A.The stereoisomers for the first compound can be represented as shown below. The one on the left is the trans-form while the other one is the cis form
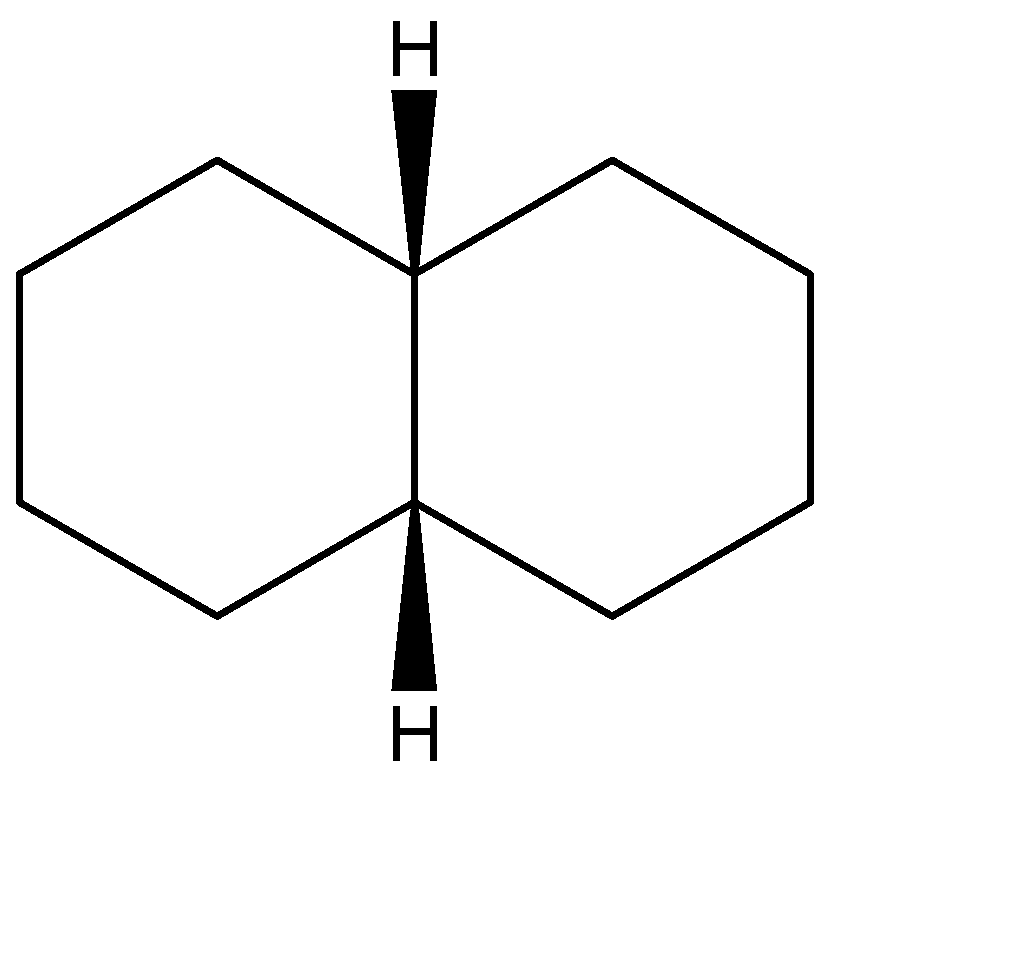
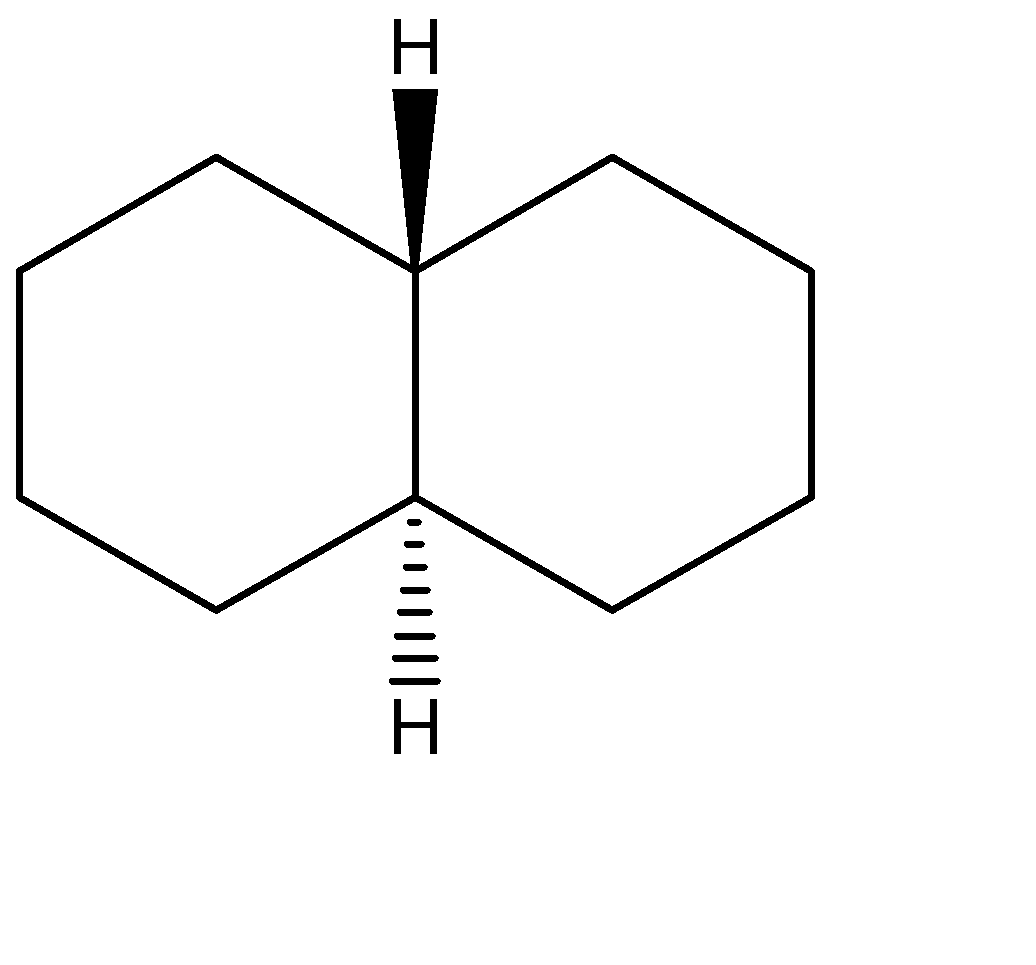
B.Similarly, the stereoisomers for the second compound can be represented as shown below. The one on the left is the trans-form while the other one is the cis form
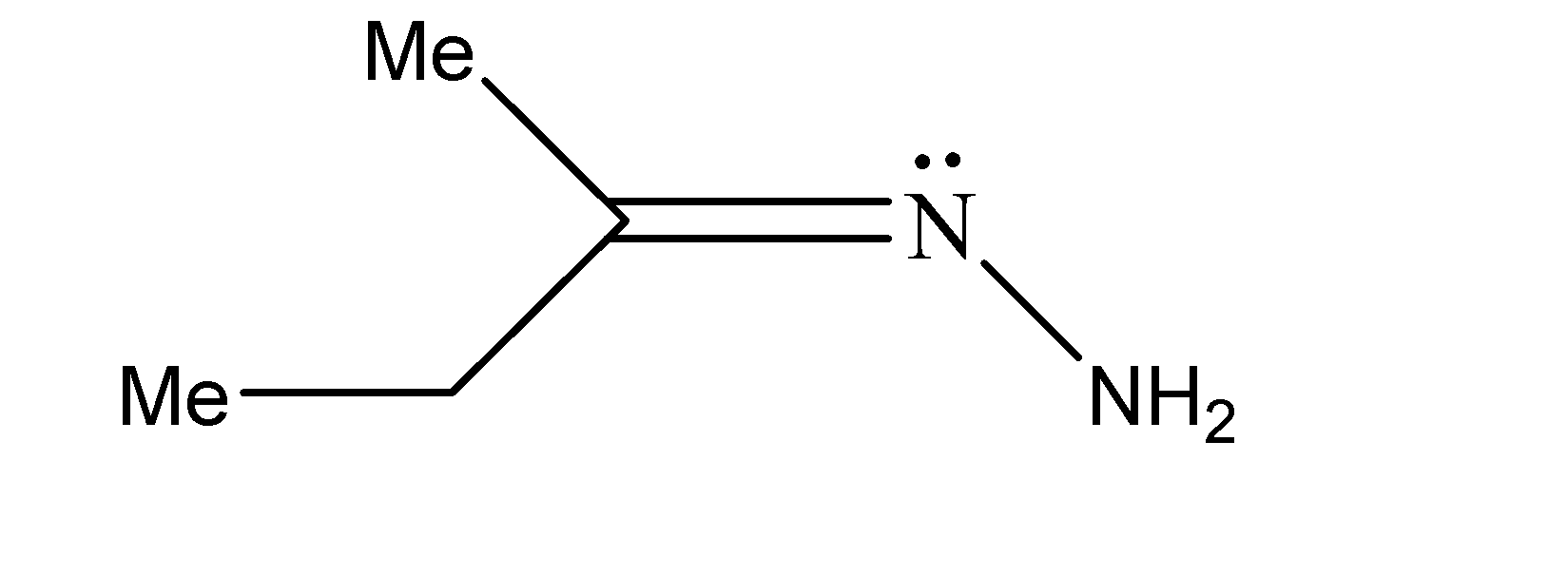
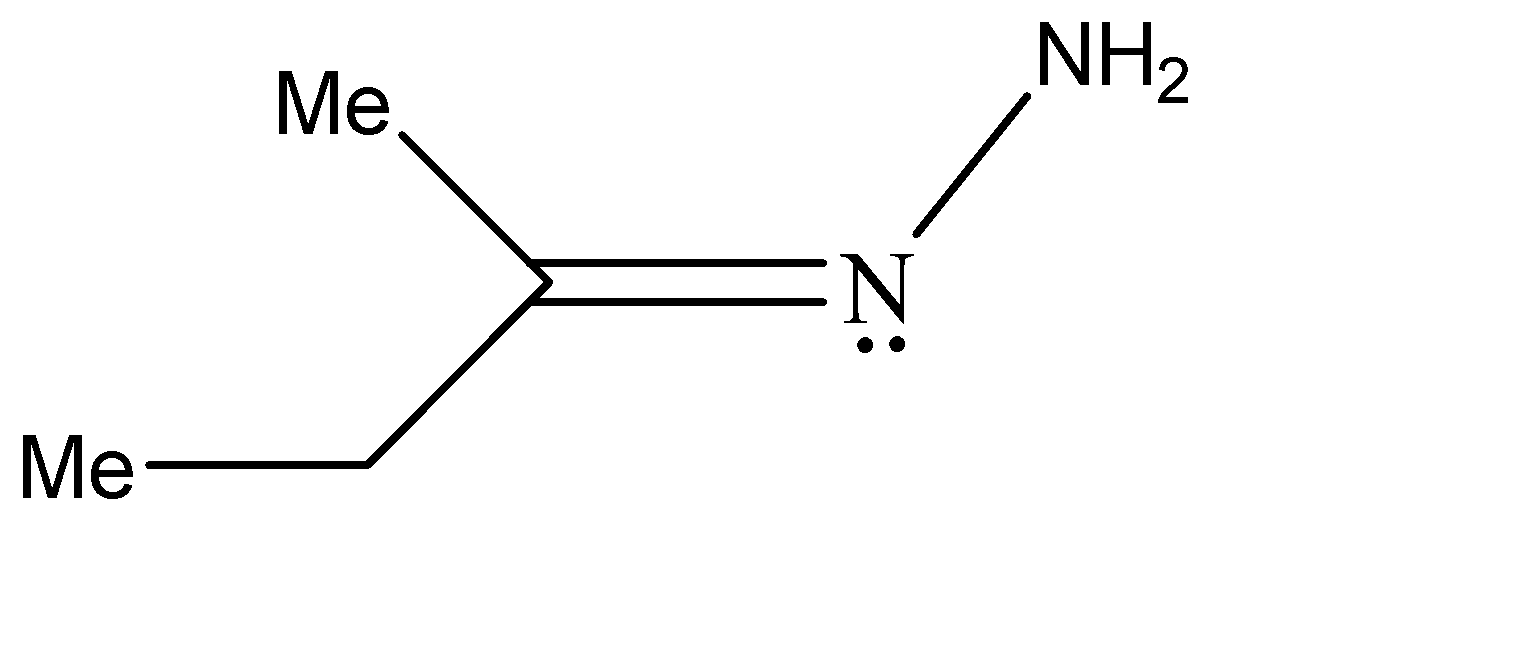
C.Similarly, the stereoisomers for the second compound can be represented as shown below. The one on the left is the trans-form while the other one is the cis form
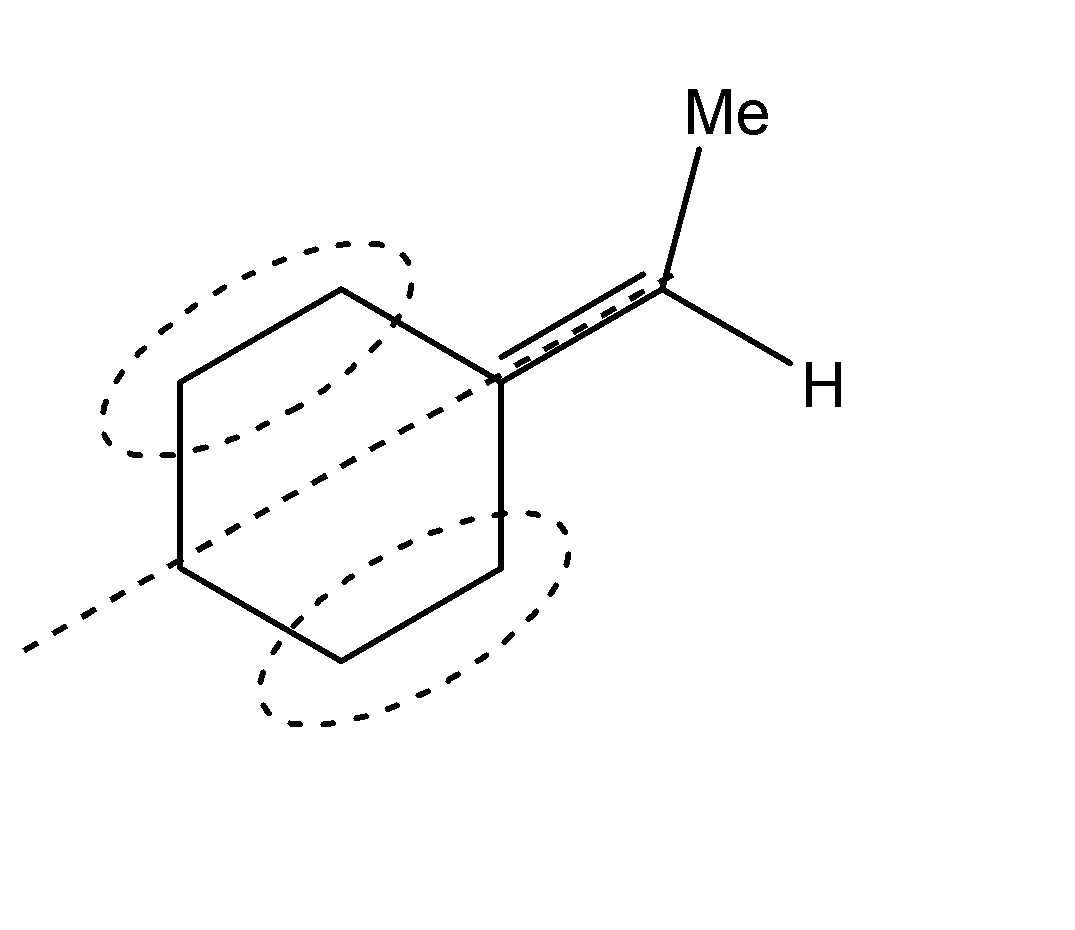
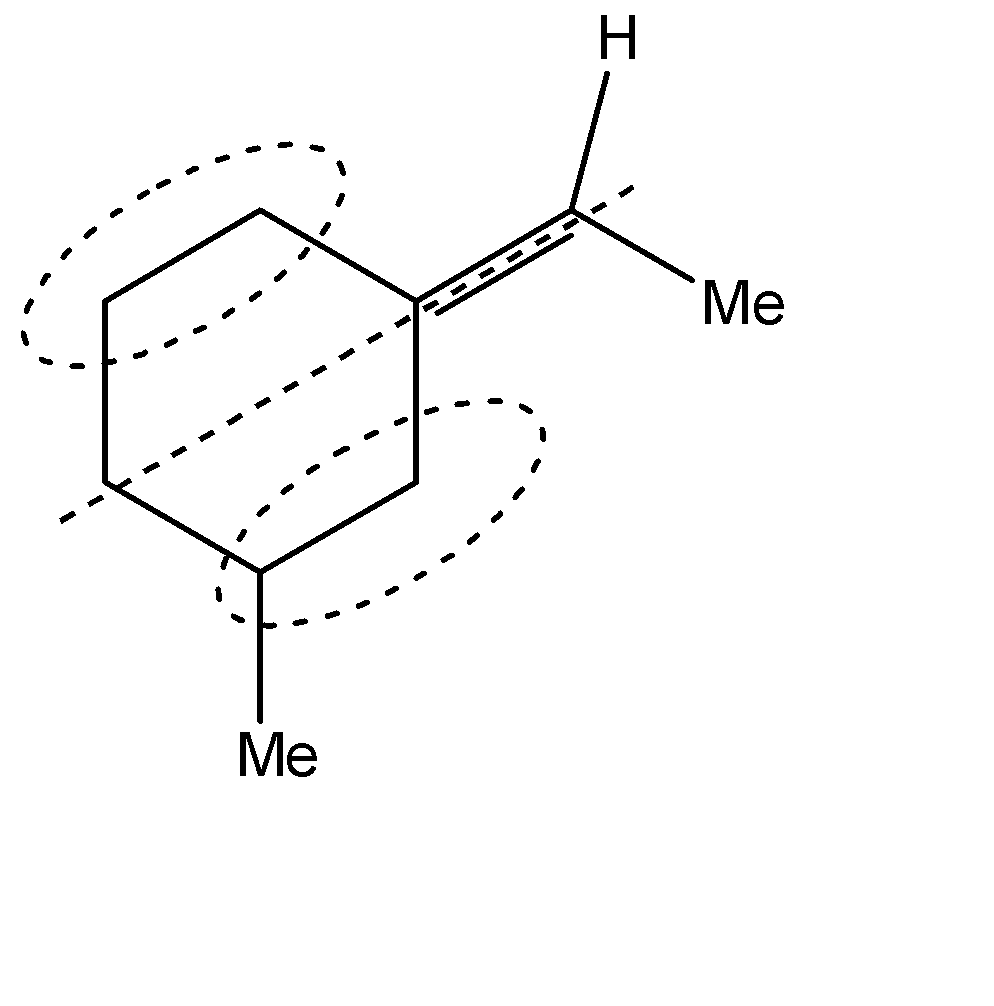
D.The compound given in the fourth option does not exhibit geometrical isomerism and hence does not exhibit stereoisomerism either.
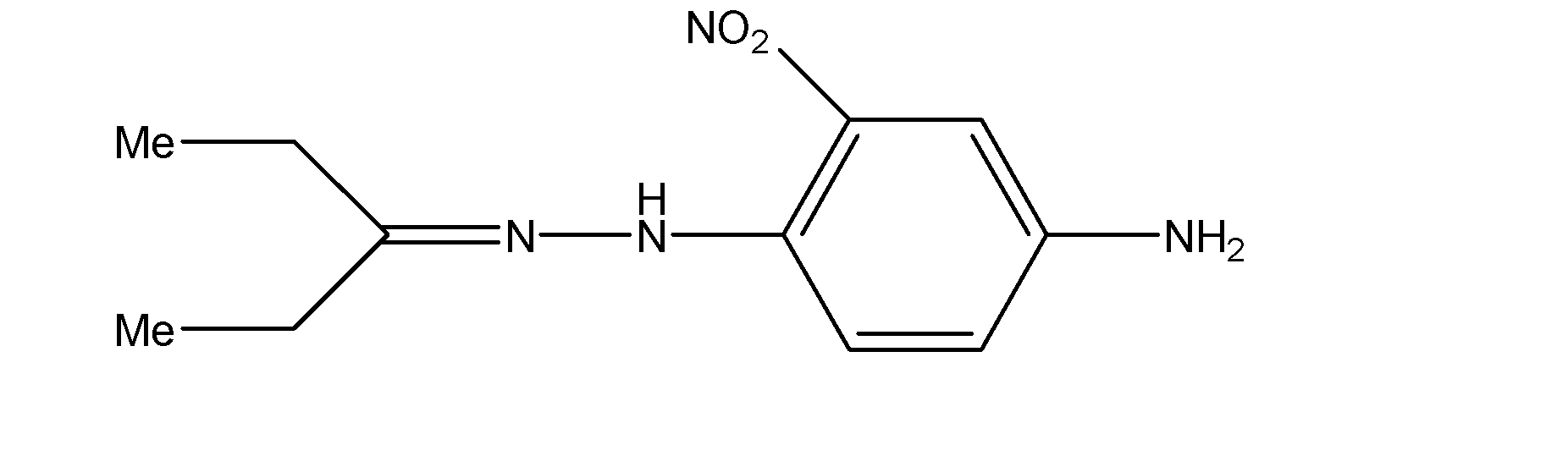
Hence, Options A, B and C are the correct options.
Note:
Enantiomers contain chiral carbons that make them non super imposable in nature and are mirror images of each other and they exist only in pairs. Enantiomers possess identical physical and chemical properties.
