Question
Question: Which of the following sequences would yield \[m - nitro\] chlorobenzene (Z) from benzene? (A) \[B...
Which of the following sequences would yield m−nitro chlorobenzene (Z) from benzene?
(A) Benzene→Cl2/FeCl3(X)H2SO4→HNO3(Z) (B)Benzene\mathop \to \limits^{{H_2}S{O_4}/HN{O_3}} (Z)(C)Benzene\mathop \to \limits^{{H_2}S{O_4}/HN{O_3}} (X)\mathop \to \limits^{C{l_2}/FeC{l_3}} (Z)$$
(D) All of these
Solution
m-nitro chlorobenzene is an organic compound having formula C6H4ClNO2. It is yellow in colour and acts as a precursor in many reactions. Benzene can be converted into m-nitro chlorobenzene by undergoing nitration and addition of chlorine.
Complete step by step answer:
As we know, benzene is C6H5, having structure as shown below
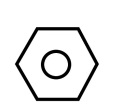
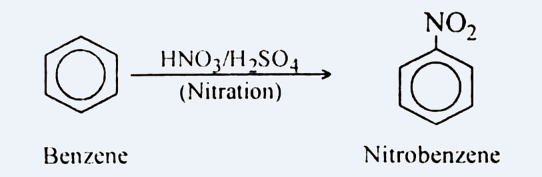
For converting it into m-nitro chlorobenzene, we need to add −nitro and −chloro groups in benzene. For the same, the reaction is carried out in two steps. In first step, we add nitrating mixture i.e. HNO3 and H2SO4 to benzene, this step is known as nitration. It leads to the addition of NO2 on Benzene. It can be shown as:
In the next step, FeCl3 and Cl2 are added to nitrobenzene. NO2 is an electron withdrawing, meta directing group. Therefore, it will add the chlorine at meta position, forming the desired product i.e. m−nitro chlorobenzene. The overall reaction is
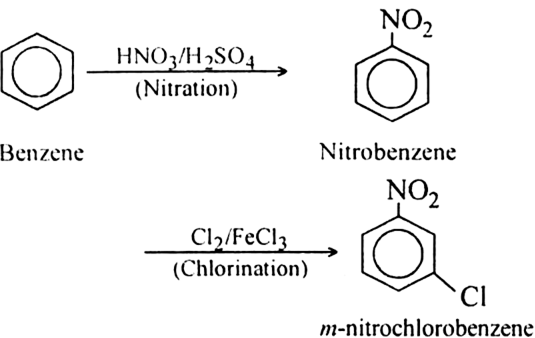
The meta isomer of nitro chlorobenzene is not active towards nucleophilic substitution at chlorine center. m-nitro chlorobenzene can be reduced to 3−chloroaniline with Fe/HCl mixture, this reaction is called Bechamp reduction.
Hence, the correct option is C.
Additional information:
m-nitro chlorobenzene is also known as 3−nitro chlorobenzene. It’s IUPAC name is 1−chloro 3−nitrobenzene . This compound generally does not have any direct application, but it acts as a precursor of other compounds such as 3−chloroaniline .
Note:
If we do the two above mentioned steps in reverse order i.e. firstly we add FeCl3 and Cl2 into Benzene, it will form chlorobenzene. In the second step, if nitration is to be done we should first note that chlorine is the ortho/para directing group. Therefore, it will add the NO2 at ortho/para position, forming o−nitro chlorobenzene or p−nitro p-nitro chlorobenzene but not m-nitro chlorobenzene.
