Question
Question: Which of the following ring compounds obeys Huckel’s rule? (A)- \({{C}_{4}}H_{4}^{-1}\) (B)- \(...
Which of the following ring compounds obeys Huckel’s rule?
(A)- C4H4−1
(B)- C4H4+1
(C)- C4H4−2
(D)- C4H4
Solution
Erich Huckel in 1931 proposed a condition for a compound to be aromatic popularly known as Huckel’s rule. It states that a cyclic, planar and conjugated system having (4n+2)π electrons, is considered to be aromatic.
Complete answer:
Aromatic compounds must obey Huckel’s rule and contain a certain number of π electrons which should be equal to 4n+2. Here, n can be zero or any other positive integer. Therefore, compounds containing2π, 6π, 10π, 14π and so on electrons are aromatic.
Let us try to find the ring compound which obeys Huckel’s rule form the above given options.
C4H4−1
Possible ring structures with C4H4−1 are shown below:
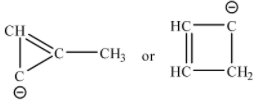
Neither of the compounds follow Huckel’s rule. In the three-membered ring compound the total number of π electrons is 4, which is not a multiple of (4n+2)π. Ring compounds containing 4π electrons are anti-aromatic in nature. In the four-membered ring, there is a sp3 hybridized tetrahedral carbon, so the ring is no longer planar. Thus, Huckel’s rule cannot be applied.
C4H4+1
Possible structure for C4H4+1 is:
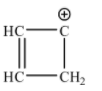
This ring is not planar as it contains a sp3 hybridized carbon. Therefore, Huckel’s rule is not applicable.
C4H4−2
With the given molecular formula the possible ring structure is shown below:
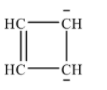
This ring compound contains 6π electrons which is equal to (4n+2)π when n=1. Therefore, it obeys Huckel’s rule and is aromatic.
C4H4
Structure of C4H4 is given as:
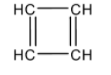
This cyclic compound contains 4π electrons and thus, it is antiaromatic. This compound also does not follow Huckel’s rule.
The only compound following Huckel’s rule is C4H4−2.
Hence, the correct option is (C).
Additional information:
Compounds which lacks one or more of the four requirements, i.e. cyclic, planar, conjugated and (4n+2)π electrons for aromatic; 4nπ for antiaromatic, to be aromatic or antiaromatic are considered as non aromatic.
Stability of the compounds follows the following order:
Aromatic > Non-aromatic> Anti-aromatic
Note:
Carefully count the number of π electrons in each compound. One lone pair of electrons is counted as 2π electrons and one double bond or triple bond in a ring also contributes 2π electrons.
