Question
Question: Which of the following resonating structure is not possible for 2, 4, 6 - trinitroiodo benzene ...
Which of the following resonating structure is not possible for 2, 4, 6 - trinitroiodo benzene
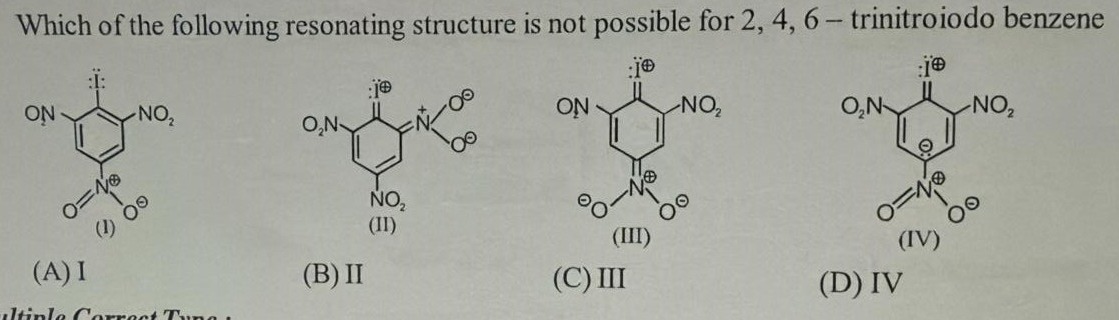
I
II
III
IV
A
Solution
The parent molecule is 2,4,6-trinitroiodobenzene. The structure is a benzene ring substituted with an iodine atom and three nitro groups at positions 2, 4, and 6.
The initial structure (similar to structure I, but with alternating double bonds in the ring) has iodine with 3 lone pairs and a single bond to the ring. The nitro groups are represented as \ce−NO2.
Let's consider the resonance forms arising from the +M effect of iodine. Iodine can donate a lone pair to the ring, forming a double bond between iodine and the ring carbon and placing a positive charge on iodine. This pushes a pi bond in the ring to the adjacent carbon, creating a negative charge. This negative charge can be at the ortho or para positions relative to iodine.
Let's draw the ring properly. Parent structure: Benzene ring with I at position 1, \ceNO2 at 2, 4, 6. Resonance forms from +M effect of I:
- I donates a lone pair to form a double bond with C1. Positive charge on I. Negative charge on C2 and C6 (ortho) and C4 (para).
Structure II shows a positive charge on I, a double bond I=C1, and a negative charge on C2. The nitro group at C2 is shown as \ce−C=N+(O−)2. This form arises from the delocalization of the negative charge on C2 into the nitro group at C2. The negative charge on C2 forms a double bond with N, and the pi bonds in the nitro group rearrange, leading to \ce−C2=N+(O−)2. This is a valid resonance form.
Structure III shows a positive charge on I, a double bond I=C1, and a negative charge on C4. The nitro group at C4 is shown as \ce−C=N+(O−)2. This form arises from the delocalization of the negative charge on C4 into the nitro group at C4. This is a valid resonance form.
Structure IV shows a positive charge on I, a double bond I=C1, and a negative charge on C6. The nitro groups are shown in their neutral form. This is a valid resonance form.
Let's look at structure I again. It shows iodine with a triple bond to the ring carbon and a positive charge on iodine, and a negative charge on the ring carbon. Iodine is in the third period and can expand its octet, but forming a triple bond with carbon is highly unlikely and unstable. The representation of a triple bond between carbon and iodine is not a typical resonance form in organic chemistry. Also, iodine typically forms single bonds with carbon in organic compounds. The representation of a triple bond to iodine with a positive charge and a negative charge on the adjacent carbon is incorrect. Iodine usually forms one single bond, or in some cases, can form double bonds in resonance structures by donating lone pairs.
Therefore, structure I is not a possible resonating structure for 2,4,6-trinitroiodobenzene.
