Question
Question: Which of the following represents the Dow process for the manufacture of phenol? 
Solution
Hint : We must remember that the Chlorobenzene is an organic aromatic compound with the chemical formulaC6H5Cl. This is colorless and due to its flammable property, it is a common solvent and a widely used intermediate to form a product.
Complete step by step solution :
We must understand that the Dow's Process is the hydrolysis of C6H5Cl (chlorobenzene) for the preparation of C6H6O(phenol). When we react C6H5Cl (chlorobenzene) with aqueous (NaOH) solution it produces (C6H5ONa) sodium phenoxide and then phenol upon reacting with H+.
In practical terms, we can prepare phenol from chlorobenzene when reacting withNaOH, in particular two steps. First, Chlorobenzene is heated with 6-8% solution of sodium hydroxide at 623K under the pressure of 200 atm to form (C6H5ONa) Sodium Phenoxide. Then Sodium Phenoxide is acidified with dil. HCl (Hydrochloric Acid) to form C6H6O (Phenol).
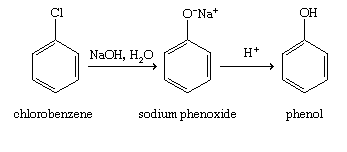
Therefore, the answer for the question is option A. :
Note : We can also use the Dow process (electrolytic method) to extract bromine from a highly concentrated NaCl solution known as brine.
In practical, bromide-containing high concentrations of Brines are treated with H2SO4(sulphuric acid) and bleaching powder to oxidize Br− (bromide) to Br2 (bromine), which remains dissolved in the water. The aqueous solution is shifted into the sacking bag and water is passed through causing bromine to volatilize freely. Bromine is trapped with iron turns to give a solution of FeBr3(ferric bromide). Treatment with more iron metal converts the FeBr3 (ferric bromide) to FeBr2(ferrous bromide).
