Question
Question: Which of the following represents the correct decreasing order of acidity of the following compounds...
Which of the following represents the correct decreasing order of acidity of the following compounds?
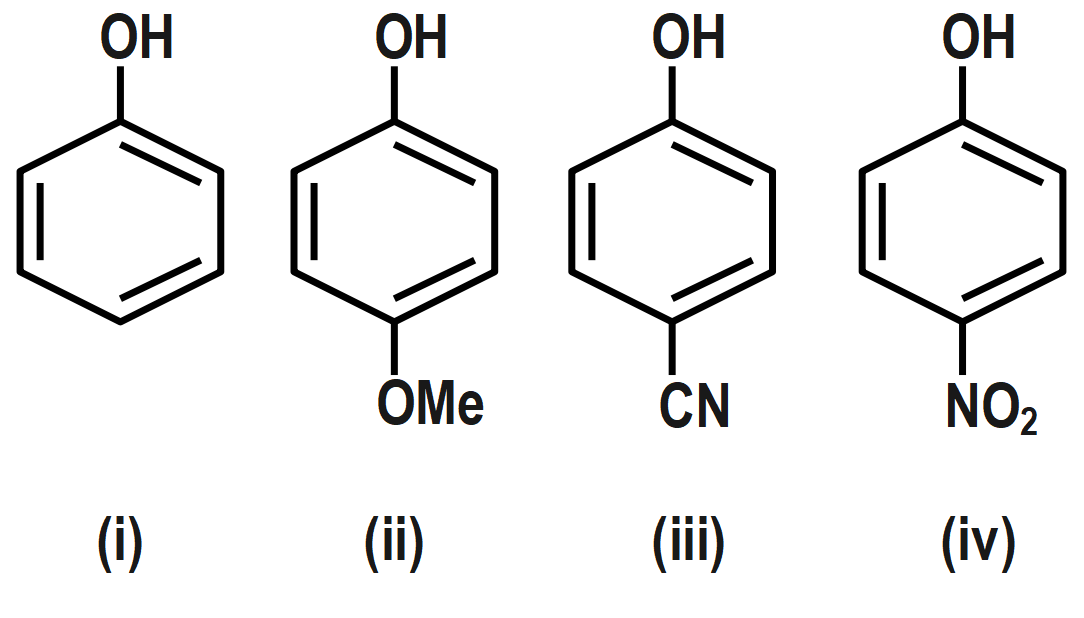
(A) ii > i > iii > iv
(B) iv > iii > ii > i
(C) iii > iv > i > ii
(D) iv > iii > i > ii
Solution
Hint : We know that the general qualities of acids might be controlled by estimating their balance constants in watery arrangements. In arrangements of a similar focus, more grounded acids ionize indeed, thus yielding higher centralizations of hydronium particles than do more fragile acids. The acid ionization constant is the equilibrium constant of an acid.
Complete Step By Step Answer:
The acidity is the measure of the donation strength of protons. The electron releasing groups decrease the acidity while the electron donating groups increase the acidity of the molecule. Here, the proton will be donated by OH group. First, let us see what acidity is and how it is affected by the addition of groups. The acidity can be defined as the measure of the capacity to donate ions. The species which can donate the hydrogen ion easily are stronger acids while those that cannot easily donate are weaker acids. In above structures, we see that hydroxyl groups will donate the ion. The other groups attached will have their effect on donation. All the groups attached are para to the hydroxyl group. The ion will be easily donated if the bonds between OH are weak.
If the electron is attracted by an oxygen atom, it will easily donate ions. Now, let us see the groups attached how they affect the donation. The electron releasing groups will give electrons and as a result, the electron density on oxygen will increase and thus, the ion will be difficult to donate. But the electron withdrawing groups will attract the electrons toward it and thus reduce electron density on the oxygen atom. As a result, the electron density on oxygen atoms will decrease and hence the ion will be easily donated. The CN group has both the effects. But overall it will withdraw electrons and thus will have electron withdrawing effect and thus, it will increase acidity in comparison to simple phenol.
The methyl group on the second molecule has electron donating effect (+I). So, it will decrease the acidity. The third molecule has a nitro group which is a strong electron withdrawing group. So, it will increase the maximum acidity. Electron withdrawing groups (−NO2 ,−CN) stabilize the phenoxide ion by dispersing the negative charge relative to phenol and increase the acidity of phenol, whereas electron donating groups (OCH3) destabilize the phenoxide ion by intensifying the negative charge relative to phenol and tend to decrease the acid strength.
Therefore, correct answer is option D i.e. the order of acidity is iv > iii > i > ii .
Additional Information:
We must remember that a combination of acidic acid and sodium acetic acid derivation on the grounds of acetic acid is more noteworthy than the Kb of its form base acetic acid derivation. It is a cushion since it contains both the feeble acid and its salt. Henceforth, it acts to keep the hydronium particle focus (and the pH) practically consistent by the expansion of either a limited quantity of a solid acid or a solid base.
Note :
Remember that the off chance that we add a base, for example, sodium hydroxide, the hydroxide particles respond with the couple of hydronium particles present. A combination of smelling salts and ammonium chloride is fundamental in light of the fact that alkali is more prominent for the ammonium particle. It is a support since it additionally contains the salt of the powerless base.
