Question
Question: Which of the following represents decreasing order of reactivity of given organic compounds towards ...
Which of the following represents decreasing order of reactivity of given organic compounds towards nitration?
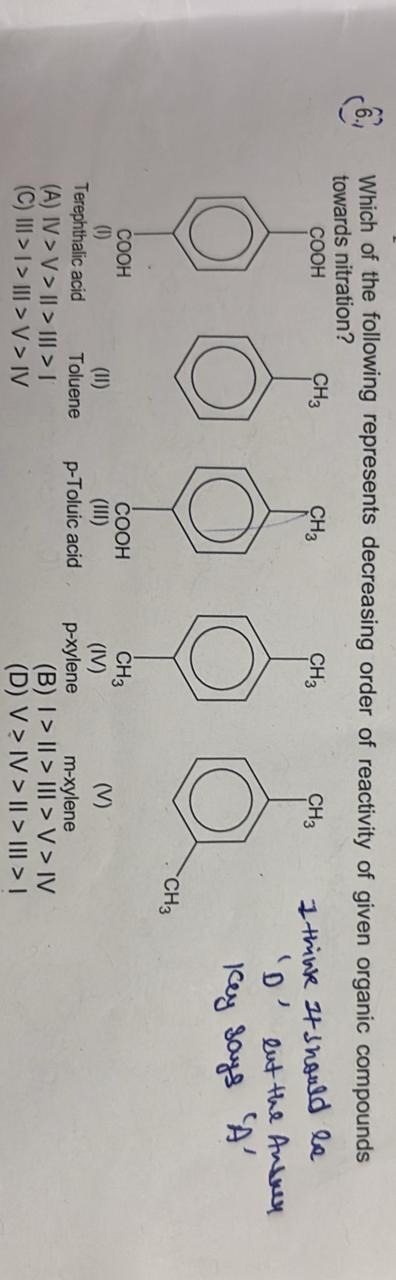
A
IV > V > II > III > I
B
I > II > III > V > IV
C
III > I > III > V > IV
D
V > IV > II > III > I
Answer
IV > V > II > III > I
Explanation
Solution
-
Substituent Effects:
- Methyl (–CH₃): +I, activates the aromatic ring.
- Carboxyl (–COOH): –I, deactivates the aromatic ring.
-
Analysis of Compounds:
- p‑xylene (IV): Two activating methyl groups (at para positions) → most activated.
- m‑xylene (V): Two methyl groups present but less efficiently synergistic than in para configuration → slightly less reactive than (IV).
- Toluene (II): One methyl group (activating) → less reactive than di‑substituted xylenes.
- p‑Toluic acid (III): One methyl (activating) and one COOH (deactivating) → net lower reactivity than toluene.
- Terephthalic acid (I): Two COOH groups (strongly deactivating) → least reactive.
-
Decreasing Order of Reactivity: → IV > V > II > III > I
