Question
Question: Which of the following represent correctly the changes in thermodynamic properties during the format...
Which of the following represent correctly the changes in thermodynamic properties during the formation of 1 mol of ideal binary solution.
A.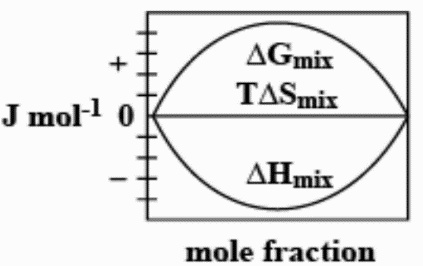
B.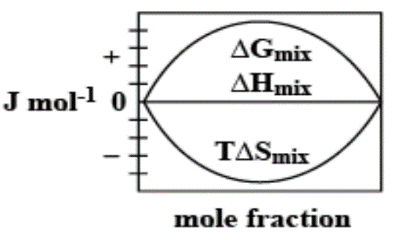
C.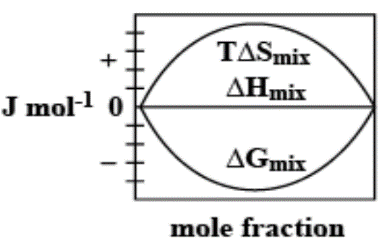
D.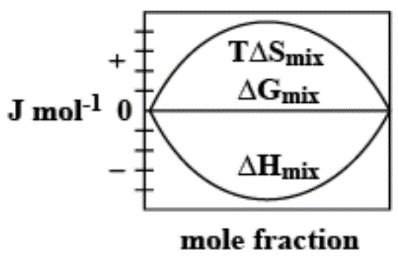
Solution
An ideal mixture is a solution in which the gas exhibits thermodynamic properties analogous to those of a mixture of an ideal gas. Mole fraction is defined as a unit of the amount of a constituent, divided by the total amount of all constituents in a mixture. A mixture of known mole fraction can be prepared by weighing off the appropriate masses of the constituents. In a mixture of ideal gases, the mole fraction can be expressed as the ratio of partial pressure to the total pressure of the mixture.
Complete step by step answer:
The enthalpy of mixing of 1 mole of ideal binary 0Jmol−10. It is known that as the mole fraction increases, the free energy change of mixing initially decreases, and it reaches a minimum value and then increases. Furthermore, with an increase in the mole fraction, the value of TΔSmixincreases and increases and finally lowers.
Thus, option C. is the right answer.
The following diagram correctly represents the changes in the thermodynamic properties during the formation of 1 mol of ideal binary solution.
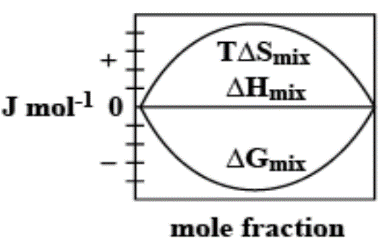
Note:
The thermodynamic properties are volume, enthalpy, and heat capacity, the entropy of mixing. If entropies are known separately for the reactants and products, then the change in entropy is just the difference and in the same way the other thermodynamic functions. The mole fraction is also called an amount fraction. It is identical to the number fraction, which is defined as the number of molecules of a constituent. The mole fraction is one way of expressing the composition of a mixture with a dimensionless quantity, mass fraction and volume fraction.
