Question
Question: Which of the following reagents would distinguish cis-cyclopenta \( - 1,2 - \) diol from the trans-i...
Which of the following reagents would distinguish cis-cyclopenta −1,2− diol from the trans-isomer?
A) Acetone
B) Ozone
C) MnO2
D) Aluminum isopropoxide
Solution
We know that cis isomers are the ones where two of the same atoms are on the same side of the double bond in a molecule whereas trans isomers are the ones which have the two atoms that lie on the opposite side of the double bond. Trans and cis isomers have the same molecular formula and molecular weight but they do differ in some aspect.
Complete step-by-step answer:
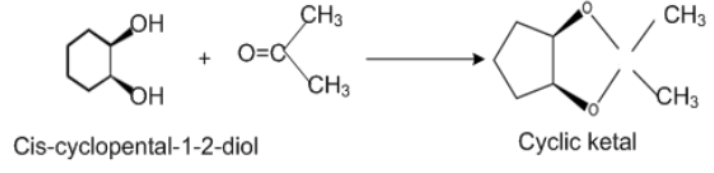
According to our question, correct answer is Acetone as with cis isomer, acetone forms bicyclic ketal. With trans- isomer, formation of the bicyclic acetal does not take place due to the presence of angle strain. Molecular formula of cis-cyclopenta −1,2− diol is: C5H10O2which contains 5 carbons, 10 hydrogen and 2 oxygen components.
Trans – isomer: A Trans isomer is an isomer where the functional groups appear on opposite sides of the double bond. Cis and Trans isomers are commonly discussed with respect to organic compounds, but they also occur in inorganic coordination complexes. Trans isomers are distinguished by adding trans-to the front of the atom's name.
Some important points about trans- isomer which are mentioned below:
Due to tightly packed molecules, the melting points of Tran’s isomers are usually higher than those of cis isomers. Trans isomers are not very polar. Many Trans isomers are non-polar molecules. The boiling point is comparatively low for Tran’s isomer as there are no strong attractive forces.
Structure of cis –cyclopenta −1,2− diol and Trans – cyclopentane −1,2− diol when react with acetone:
Cis-cyclopenta −1,2− diol when reacts with Acetone, structures of cyclic ketal are formed while trans-isomers of cyclopenta −1,2− diol can't form cyclic ketal.
Note: Cis isomers are always polar whereas the trans isomers are mainly non-polar. We know that the atoms are very loosely packed in the cis isomers so that is why they have low melting points than trans isomers. Also, Trans isomer is less acidic than cis isomer as trans isomers do readily emit the protons.
