Question
Question: Which of the following reagents may be used to distinguish between phenol and benzoic acid? [A] Aq...
Which of the following reagents may be used to distinguish between phenol and benzoic acid?
[A] Aqueous NaOH
[B] Tollen’s reagent
[C] Molisch reagent
[D] Neutral FeCl3
Solution
To answer this you must know which groups are the following reagents used to identify. Tollen’s test is given by aldehydes and alkynes. Carbohydrates give Molisch’s test. You can use this to find out the appropriate test to distinguish between phenol and benzoic acid.
Complete step by step solution:
Firstly let us draw the structures of benzoic acid and phenol.
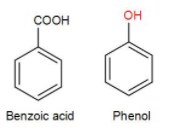
Now, let us discuss the tests mentioned in the options.
Firstly, we have aqueous NaOH. Benzoic acid will react with it and will result in formation of sodium benzoate through the formation of a benzoate ion. Similarly, phenol reacts with sodium hydroxide and gives a phenoxide ion. Both solutions are colourless so we cannot use it to determine the two. Then we have Tollen’s test. Tollen’s test is also known as the silver mirror test. We use it to determine the presence of aldehyde and aromatic aldehydes. It is also used for the detection of some alpha-hydroxy ketones which undergo tautomerization to form an aldehyde. Neither benzoic acid nor phenol gives this test. So, we cannot use this test either. Then we have Molisch reagent. It is alpha naphthol i.e. C10H8OH dissolved in ethanol. We use this reagent to perform the Molisch’s test for identification of carbohydrates. Neither benzoic acid nor phenol gives this test. And lastly we have neutral FeCl3. Phenol reacts with FeCl3 to give us a violet coloured iron - phenol complex whereas benzoic acid gives us a precipitate of ferric benzoate. The precipitate is buff coloured therefore, we can differentiate phenol and benzoic acid from ferric chloride test.
