Question
Question: Which of the following reactions would give \( trans - \) alkene. 
A. (I)
B. (II)
C. (I), (III)
D. (II), (IV)
Solution
Hint : An optically inactive molecule whose molecule is superimposable on its mirror image in spite of the presence of asymmetric carbon atoms is known as meso compound. Such a molecule can be recognized by the fact that it possesses a mirror plane which divides the molecule into two halves which are mirror images of each other. Optical inactivity of a meso compound is due to internal compensation.
Complete Step By Step Answer:
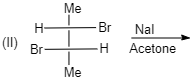
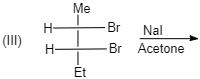
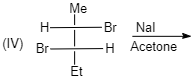
cis -form indicates that the substituents are on the same side of some plane (adjacent positions) and Trans-form indicates that the substituents are on the opposite positions.
Meso compounds having two same groups’ present with Anti-elimination gives trans -alkene.
Racemic compound having two same groups in Anti-elimination gives cis -alkene.
Racemic compounds with two different groups but the two eliminating groups present in anti-position on anti-elimination gives Trans-alkene as a product.
Racemic compound with two different groups but the two eliminating groups present in syn-position on anti-elimination gives cis -alkene.
(I)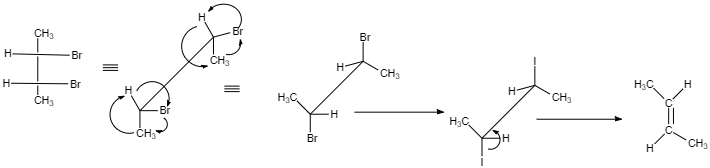
(II)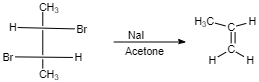
(III)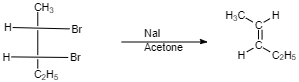
(IV)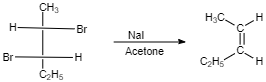
Therefore the correct answer is option C.
Note :
When there are three or four different groups attached to the carbon atoms of a double bond, it becomes difficult to assign cis and trans designation to isomers, to overcome this problem, a general system of geometrical isomers called the E and Z system was framed.
If the two higher prioritized groups are on the same side of the double bond, the isomer is called Z and if the two higher prioritized groups are on opposite sides of the double bond, the isomer is called E.
