Question
Question: Which of the following reactions with chloroform will give chloretone? (a). \(HN{{O}_{3}}\) (b)....
Which of the following reactions with chloroform will give chloretone?
(a). HNO3
(b). (CH3)2C=O
(c). Chloral
(d). (CH3)2CHCHO
Solution
The reaction of acetone with chloroform results in the production of a hypnotic drug, chloretone. This reaction is an exothermic as well as a vigorous reaction. Also, it requires a strong basic medium
Complete step by step answer:
At first, what is chloroform? Chloroform is a colorless, volatile, liquid derivative of dichloromethane with ether-like odor.
It was used as an anesthetic during surgeries. But nowadays, the primary use of chloroform is in the production of refrigerant Freon.
The chemical formula is CHCl3
In the first option, HNO3 is given. The chloroform on reaction with HNO3 (nitric acid) produces chloropicrin that is used as tear gas (war gas) or it can also be used as an insecticide.
HNO3+CHCl3→CCl3NO2+H2O (chloropicrin)
Talking about the second option, it is acetone (CH3)2C=O. When acetone reacts with chloroform it result in the formation of chloretone (Chlorobutanol)
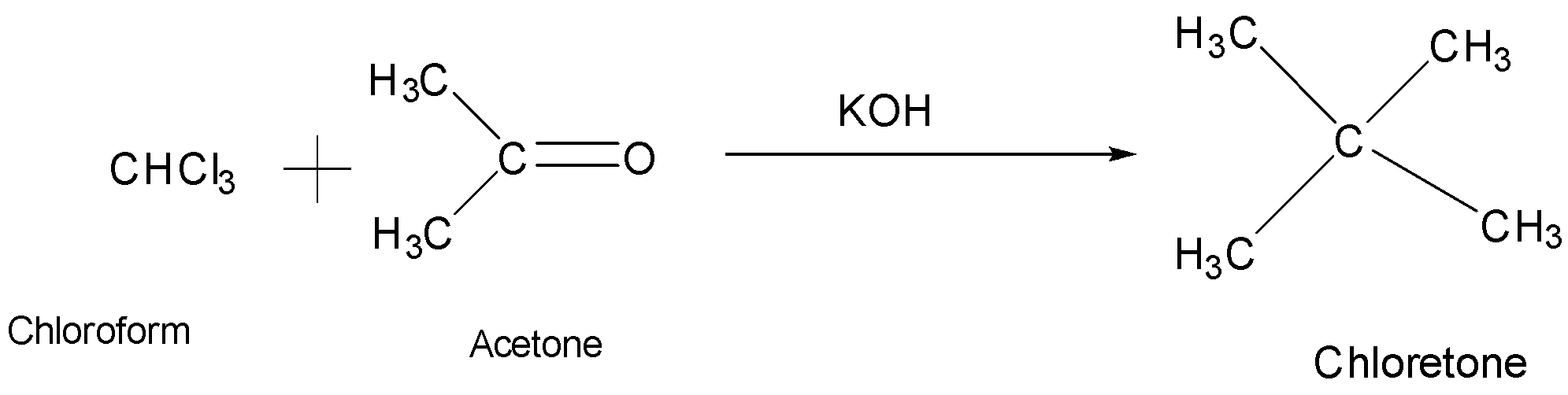
So, the acetone with chloroform produces chloretone.
So, the correct answer is Option B.
Additional Information:-
Chloretone is also known as Chlorobutanol and is a hypnotic drug. It is sedative, hypnotic and weak anesthetic. It has antibacterial and antifungal properties.
Note: The reaction of acetone and chloroform produces chloretone in basic medium such as in the presence of KOH only. Moreover, chloretone is known to have slept producing properties. It is also used as a common detergent preservative in eye drops and other ophthalmic therapeutic formulations.
